Vaccine AdjuvantsMarket Overview
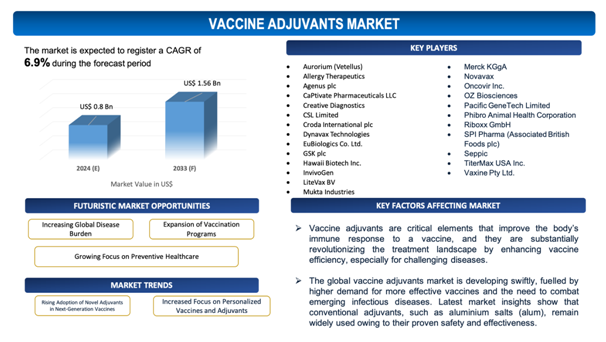
The global vaccine adjuvants market is estimated to be worth over USD 1.56Bn in 2033 and is expected to grow at CAGR of 6.9% during the forecast period (2024-2033).
Vaccine adjuvants are criticalelements that improve the body’s immune response to a vaccine, and they are substantiallyrevolutionizing the treatment landscape by enhancing vaccine efficiency, especially for challenging diseases. Conventionally, vaccines depend on attenuated or inactivated pathogens to trigger an immune response. However, some vaccines, particularly those based on subunit or recombinant antigens, may not stimulate a strong enough immune reaction on their own. This is where adjuvants come in—by accelerating and directing the immune system’s response, they increase the effectiveness of vaccines, helping the body to build stronger and longer-lasting immunity.
Adjuvants like aluminium salts (alum), oil-in-water emulsions, and newer formulations such as liposomes, saponins, and toll-like receptor (TLR) agonists are now being incorporated into vaccine development to battle diseases like influenza, hepatitis, HPV, and even emerging infectious threats like COVID-19. These adjuvants not only help create a better initial immune response but also minimize the amount of antigen required per dose, enabling for improved vaccine production efficiency. In addition, they can provoke both humoral (antibody-mediated) and cellular immunity, offering a more comprehensive protection against infections.
Latest advancements in adjuvant technology are also holding a crucial role in the development of vaccines for more complex and difficult-to-treat diseases, such as cancer and HIV, where conventional vaccine strategies have been less effective. By enhancing the body’s recognition of specific antigens and encouraging a more tailored immune response, adjuvants are allowing the creation of more effective vaccines. This transformative approach is crucial in modern immunology, as it opens new avenues for tackling a wider range of diseases and emerging pathogens with greater safety, efficacy, and long-term protection.
Figure 1. Vaccine Adjuvants: Market Size

Get more details on this report - Request Free Sample
Key Market Insights
The global vaccine adjuvants market is developingswiftly, fuelled by higher demand for more effective vaccines and the need to combat emerging infectious diseases. Latest market insights show that conventional adjuvants, such as aluminum salts (alum), remain widely used owing to their proven safety and effectiveness. However, the market is experiencing a transition towards more advanced adjuvant systems like oil-in-water emulsions (e.g., MF59), liposomes, saponins (e.g., QS-21), and toll-like receptor (TLR) agonists. These novel adjuvants offer improved immunogenicity and are critical in developing vaccines for challenging diseases such as HIV, cancer, and malaria.
One of the most notable recent developments is the incorporation of adjuvants into vaccines for emerging diseases, as seen with COVID-19 vaccines. The usage of advanced adjuvants has enhanced vaccine efficiency by encouraging stronger immune responses and improving durability, which is especiallysignificant for subunit vaccines. Moreover, adjuvants are minimizing the antigen dosage required, enhancing manufacturing scalability and accessibility, particularly in resource-limited regions.
In terms of novel technologies, nanoparticle-based adjuvants are gaining momentum, offering the capability to deliver antigens and adjuvants simultaneously, enhancing targeting, and improving both humoral and cellular immunity. Furthermore, there is continuous research into personalized adjuvants that cater to individual immune system variations, marking a shift toward personalized vaccine strategies.
Geographically, North America and Europe dominate the vaccine adjuvants market attributing to robust R&D investments and advanced healthcare infrastructure, while Asia-Pacific is emerging as a high-growth region fuelled by growing healthcare demand and government initiatives.
Market Dynamics
Market Drivers
Growing Focus on Preventive Healthcare
The growing focus on preventive healthcare is a notable driver for the growth of the global vaccine adjuvants market. As healthcare systems globallymove from treatment-focused models to prevention-centric approaches, there is soaringfocus on vaccines as a crucial tool for disease prevention. This transition is fuelled by the increasing burden of infectious diseases, emerging health threats, and the necessity to minimize healthcare costs associated with treating chronic conditions. Vaccine adjuvants hold a critical role in improving the efficiency of vaccines, making them more effective in preventing diseases, which streamlines with the worldwide focus on preventive health.
Governments and healthcare organizations are encouraging immunization programs to avoid outbreaks of diseases such as influenza, HPV, hepatitis, and more recently, COVID-19. The use of advanced adjuvants in vaccines helps improve immune responses, especially in populations with weaker immune systems, such as the elderly, infants, and immunocompromised individuals. This is particularly important as global populations age and become more vulnerable to infections.
Other than that, preventive healthcare initiatives are being reinforced by increasing public awareness and government policies focused at minimizing healthcare expenditures through proactive disease management. Adjuvants improve the potency and longevity of immune responses, allowing vaccines to be more efficient with fewer doses and lower antigen quantities, which makes them more scalable and cost-effective. This soaring demand for efficient and durable vaccines in preventive healthcare is driving investments in innovative adjuvants, accelerating market growth, and revolutionizing the landscape of global immunization efforts.
Market Restraints
With regard to numerous advantages of vaccine adjuvants, the market faces several challenges due to the unique characteristics and requirements associated with these potent pharmaceutical products. Some of the key market challenges include:
- High Development Costs and Complex Regulatory Approvals: Developing new vaccine adjuvants involves significant R&D investment, as well as complex and lengthy clinical trials to ensure safety and efficiency. The stringent regulatory requirements imposed by health authorities like the FDA and EMA further complicate the approval process, delaying the commercialization of novel adjuvants and increasing the overall cost of bringing them to market.
- Adverse Side Effects and Safety Concerns: While adjuvants improve the immune response, there are concerns about potential side effects, such as allergic reactions, local inflammation, and systemic responses. These safety issues, particularly in certain populations (e.g., the elderly or immunocompromised), can deter the adoption of adjuvanted vaccines and pose a challenge to market growth, as they may result in reluctance among healthcare providers and patients.
Get more details on this report - Request Free Sample
Market Opportunity
Technological Advancements in Vaccine Manufacturing
Technological advancements in vaccine manufacturing present a major market opportunity for the growth of the global vaccine adjuvants market. Advancements such as mRNA platforms, recombinant DNA technology, and nanoparticle-based delivery systems are transforming how vaccines are developed and produced, enabling for more accurate and effective formulations. These technologies allow the incorporation of advanced adjuvants that improve immune responses, making vaccines more potent, particularly in cases of subunit or protein-based vaccines that are likely to require adjuvants to elicit a strong immune reaction.
The transition towards next-generation vaccines, such as mRNA vaccines, opens new horizons for adjuvant use, as these vaccines often need novel adjuvant systems to boost immune responses. In addition, innovations in biomanufacturing techniques, such as single-use bioreactors and continuous manufacturing, are improving the scalability and cost-efficiency of vaccine production. This makes it simpler to produce adjuvanted vaccines on a large scale, addressing global healthcare needs more effectively.
As vaccine manufacturing becomes more sophisticated and efficient, the demand for adjuvants that can optimize these advanced vaccines is estimated to grow, creating a strong market opportunity for innovative adjuvant solutions that improvesafety, efficacy, and distribution scalability.
Market Trends
- Rising Adoption of Novel Adjuvants in Next-Generation Vaccines: As new vaccine platforms, such as mRNA and viral vector vaccines, gain significance, there is anincreased trend toward incorporating novel adjuvants like saponins, liposomes, and toll-like receptor (TLR) agonists. These adjuvants are proving critical in improving immune responses and enhancing the efficiency of vaccines for diseases like COVID-19, cancer, and malaria, driving further innovation in the market.
- Increased Focus on Personalized Vaccines and Adjuvants: The global vaccine adjuvants market is experiencing a shift toward personalized vaccine approaches, where adjuvants are customized to an individual’s immune profile. With advancements in immunology and precision medicine, the trend is to design adjuvants that cater to specific populations, such as the elderly or immunocompromised, to optimize vaccine effectiveness and safety for diverse groups. This is creating new avenues for specialized adjuvant development.
Vaccine Adjuvants Market: Key Segments
By Product
- EmulsionsAdjuvants
- PathogenAdjuvants
- Saponin-based Adjuvants
- ParticulateAdjuvants
- Other Adjuvants
By Route of Administration
- Subcutaneous
- Intramuscular
- Other Route of Administration
By Disease Type
- Infectious
- Cancer
- Others
By Application
- Research Applications
- Commercial Applications
By Type
- Human Vaccine Adjuvants
- Veterinary VaccineAdjuvants
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Figure 4. Vaccine Adjuvants Market: Distribution by Region

Get more details on this report - Request Free Sample
Vaccine Adjuvants Market: Regional Analysis
North America is expected to dominate the vaccine adjuvants market owing to the well-established veterinary healthcare infrastructure. Asia-Pacific is projected to score the highest growth rate and exhibit the highest CAGR for the forecast period. This is because of the rising expenditure to develop veterinary healthcare infrastructure and the increased focus of the government on immunization programs.
Leading Vaccine Adjuvants Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Vaccine Adjuvantsmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Vaccine Adjuvantsmarket are briefly discussed below.
The report includes the list of players operating in the global Vaccine Adjuvantsmarket. Some of the key players include:
- Aurorium (Vetellus)
- Allergy Therapeutics
- Agenus plc
- CaPtivate Pharmaceuticals LLC
- Creative Diagnostics
- CSL Limited
- Croda International plc
- Dynavax Technologies
- EuBiologics Co. Ltd.
- GSK plc
- Hawaii Biotech Inc.
- InvivoGen
- LiteVax BV
- Mukta Industries
- Merck KGgA
- Novavax
- Oncovir Inc.
- OZ Biosciences
- Pacific GeneTech Limited
- Phibro Animal Health Corporation
- Riboxx GmbH
- SPI Pharma (Associated British Foods plc)
- Seppic
- TiterMax USA Inc.
- Vaxine Pty Ltd.
Vaccine Adjuvants Market: Key Developments
- In August 2024, Novavax, Inc., a global company advancing protein-based vaccines with its Matrix-M™ adjuvant, announced the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for active immunization to prevent COVID-19 in individuals aged 12 and older. Novavax's vaccine is included in the recommendations issued by the U.S. Centers for Disease Control and Prevention (CDC) on June 27, 2024.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
|
Key Report Attributes |
Details |
||
|
|
Base Year |
2023 |
||
|
|
Forecast Period |
2024-2033 |
||
|
|
CAGR (2024-2033) |
6.9% |
||
|
|
Product |
|
||
|
|
Route of Administration |
|
||
|
|
Disease Type |
|
||
|
|
Application |
|
||
|
|
Type |
|
||
|
|
Key Geographical Regions |
|
||
|
Key Companies Profiled |
|
|
||
