Pediatric Growth Hormone Deficiency Market Overview
The global pediatric growth hormone deficiencymarket is estimated to be worth over USD 13.96Bn in 2033 and is expected to grow at CAGR of15.0% during the forecast period (2024-2033).
Pediatric Growth Hormone Deficiency (PGHD) can be understood as a condition characterized by inadequate production of growth hormone (GH) during childhood and adolescence, hindering normal growth and development. Growth hormoneholds a crucial role in exciting growth, specifically in bones and muscles, and governsdifferent metabolic processes critical for overall health. PGHD are likely toarise from varied causes, comprising genetic mutations, congenital conditions affecting the brain tumours, pituitary gland, or brain injuries. Clinically, PGHD are likely to manifest as short stature, delayed puberty, or other developmental delays, impacting both physical and psychosocial well-being.
The global market for pediatric growth hormone deficiency surrounds a gamut of therapeutic interventions focused at amending hormone deficiencies and fostering normal growth trajectories in affected individuals. Primary treatment centres on the administration of synthetic GH through subcutaneous injections, spurring growth and ameliorating height deficits and body composition abnormalities. Complementary therapies, such as nutritional counselling and psychosocial support, are likely to complement GH therapy to maximize outcomes and address related challenges.
Numerous factors define the dynamics of the pediatric growth hormone deficiency market. Progressions in diagnostic methodologies and greater awareness among healthcare providers and caregivers contribute to earlier detection and intervention. This, along with diversifying access to treatment options across the globe, both in developed and developing regions, fuels market growth. Along with that, continuing research and development efforts emphasize on improving the efficacy, safety, and convenience of GH therapies. Novel formulations, comprising long-acting GH preparations, and innovative delivery devices represent substantial developments in the field.
However, challenges continue, comprising the increased cost of GH therapy, disparities in access to specialized care, and the need for sustained education and support for patients and caregivers. Regardless of these barriers, coordinated efforts from healthcare stakeholders, favourable reimbursement policies, and government initiatives emphasized at supporting early intervention and access to care hold promise for addressing these challenges. As a result, the global market for pediatric growth hormone deficiency is poised for continued growth, offering commitment for enhanced outcomes and enhanced quality of life for affected children and their families
Figure 1. Pediatric Growth Hormone Deficiency: Market Size
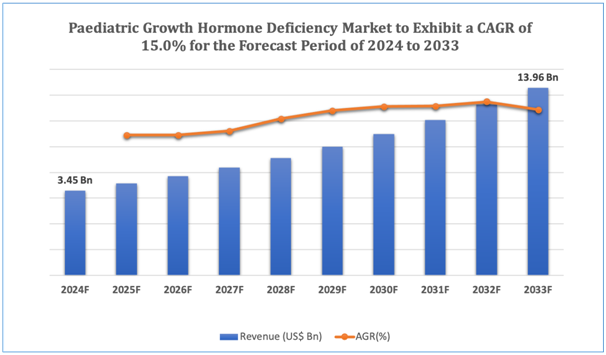
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global pediatric growth hormone deficiency (PGHD) market is characterized by key insights and a dynamic landscape shaped by significant developments and innovative technologies. One notable insight is the increasing prevalence of PGHD worldwide, driven by factors such as genetic predispositions, congenital abnormalities, and improvements in diagnostic capabilities. This growing prevalence underscores the importance of early detection and intervention in optimizing outcomes for affected children. In terms of the market landscape, synthetic growth hormone (GH) therapies remain the cornerstone of PGHD management, with subcutaneous injections being the primary mode of administration. Major players in the pharmaceutical industry offer a variety of GH formulations, including standard daily injections and long-acting preparations, to address different patient needs and preferences. Additionally, supportive therapies such as nutritional counselling and psychological support complement GH treatment to enhance overall outcomes.
Recent developments in the global PGHD market include advancements in GH formulations and novel delivery technologies focused at enhancing treatment efficiency and patient adherence. Long-acting GH preparations provide the benefit of less frequent dosing schedules, mitigating the burden of daily injections for patients and caregivers. Along with that, innovative delivery devices, such as autoinjectors and needle-free systems, improve convenience and ease of administration, particularly for pediatric patients. Another major development is the integration of digital health solutions, such as telemedicine platforms and mobile applications, to streamline patient monitoring and facilitate remote access to specialized care. These technological advancements not only enhance patient engagement but also allow healthcare providers to monitor treatment adherence and adjust therapy regimens as needed. Overall, the global PGHD market continues to evolve with advancements in treatment alternatives and innovative technologies, fuelled by a soaring understanding of the condition and a commitment to enhancing outcomes for affected children.
Market Dynamics
Market Drivers
Increasing Incidence of Pediatric Growth Hormone Deficiency
There is a soaring recognition of PGHD as a common endocrine disorder affecting children across the world. According to National Institutes of Health, the prevalence of GHD is anticipated at approximately 1:4,000 to 1:10,000 (1–4). The diagnosis of GHD together with other pituitary deficiencies (i.e., multiple pituitary hormone deficiency, MPHD) and/or organic pathologies such as central nervous system (CNS) tumor is usuallyforthright.Factors contributing to this upsurge in incidence compriseenhanced diagnostic techniques, escalated awareness among healthcare providers, and growing prevalence of risk factors such as genetic predispositions and congenital abnormalities. Since more children are diagnosed with PGHD at earlier stages, there is a higher demand for effective treatment alternatives to address growth deficits and associated developmental delays. This growing incidence not only diversifies the patient population but also underlines the pressing need to optimize treatment strategies and enhance patient outcomes. As a consequence, pharmaceutical firms and research institutions are intensifying efforts to develop novel therapies and innovative treatment modalities tailored to the specific needs of pediatric patients with GH deficiency. In addition, supportive government initiatives and favorable reimbursement policies further stimulate market growth by supporting access to specialized care and treatment options for affected children. Overall, the growing incidence of PGHD represents a compelling market opportunity, fueling innovation, investment, and advancements in the global pediatric growth hormone deficiency market.
Market Restraints
With regard to numerous advantages of Pediatric Growth Hormone Deficiency, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- High Cost of Treatment:The increased cost of growth hormone (GH) therapy presents a major restraint on the global pediatric growth hormone deficiency market.GH therapy comprises prolonged treatment durations and frequent injections, resulting in substantial financial burdens for patients and healthcare systems.Limited reimbursement coverage and disparities in access to affordable treatment options further aggravate the financial challenges suffered by patients and caregivers, hampering market growth and adoption of GH therapy.
- Adverse Effects and Safety Concerns:Side effects associated with growth hormone (GH) therapy, such as fluid retention, injection site reactions, and potential long-term risks, represent a substantial market restraint.Safety concerns encompassing GH therapy, specifically in pediatric populations, raise apprehensions among patients, caregivers, and healthcare providers, influencing treatment adherence and acceptance.The need for regular monitoring and management of potential side effects adds complexity to treatment regimens, leading to patient reluctance and healthcare provider hesitation, thereby confining market growth and adoption of GH therapy.
Market Opportunities
Strategic Collaborations and Partnerships
Strategic collaborations and partnerships exhibit a persuasive market opportunity for the global pediatric growth hormone deficiency market. Since the demand for advanced therapies and treatment solutions persists to surge, collaboration between pharmaceutical firms, biotechnology companies, research institutions, and healthcare organizations provides a pathway to propel research and development efforts, improve treatment alternatives, and expand market reach. Collaborative ventures allow the pooling of resources, expertise, and intellectual property, supporting the discovery and development of novel growth hormone (GH) therapies and delivery technologies. In addition to that, partnerships with academic institutions and research centers offer access to cutting-edge scientific advancements and clinical expertise, fostering the translation of scientific discoveries into clinically meaningful treatments. What is more, collaborations with regulatory agencies and patient advocacy groups can streamline the regulatory approval process, address unmet medical needs, and raise awareness about pediatric growth hormone deficiency (PGHD) among healthcare providers and the general public. By leveraging the cohesive strengths and resources of multiple stakeholders, strategic collaborations and partnerships stimulate innovation, increase market competitiveness, and enhance patient access to advanced therapies and specialized care. Overall, fostering collaborative relationships within the pediatric growth hormone deficiency market not only stimulates progress in acknowledging the needs of pediatric patients with GH deficiency but also cultivates a supportive ecosystem that supportspersistent advancement and improvement in patient outcomes.
Market Trends
- Increasing Emphasis on Personalized Medicine:There is anevident trend towards personalized medicine approaches in the management of pediatric growth hormone deficiency (PGHD), led by advancements in genetic testing and biomarker identification. Healthcare providers are more and more utilizing genetic testing and molecular profiling to tailor treatment regimens to the particular needs and genetic makeup of individual patients. As personalized medicine gains prominence, the global PGHD market is experiencing a shift towards individualized treatment strategies that prioritize patient well-being and enhance treatment efficiency.
- Adoption of Digital Health Solutions:There is a soaring trend towards the adoption of digital health solutions in the management of pediatric growth hormone deficiency (PGHD), aimed at improving patient engagement, treatment adherence, and remote monitoring.Mobile applications, telemedicine platforms, and wearable devices enable real-time tracking of treatment progress, medication adherence, and growth parameters.These digital tools promote communication between patients, caregivers, and healthcare providers, supportingcollective decision-making and enhancing continuity of care.

Get more details on this report - Request Free Sample
Pediatric Growth Hormone Deficiency Market: Key Segments
By Type
- Acquired
- Congenital
- Idiopathic
ByTreatment
- Growth Hormone Shots
- Others
By Diagnosis
- Blood tests
- X-Ray
- CT Scan
- MRI
- GH Stimulation Test
- Others
By Symptoms
- Younger-Looking Face
- Delayed Puberty
- A chubby Body Build
- Impaired Hair Growth
- Others
By Dosage
- Injection
- Others
By Route of Administration
- Subcutaneous
- Others
By End user
- Clinic
- Hospital
- Others
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Pediatric Growth Hormone Deficiency Market: Regional Analysis
North America is expected to dominate the market due to the presence of major key players, high disposable income, and well-developed healthcare infrastructure in this region. Asia-Pacific is expected to show the fastest growth during the forecast period due to the increasing research and development activities, rising investment in the healthcare sector, and growing government support.
Figure 4. Pediatric Growth Hormone Deficiency Market: Distribution by Region
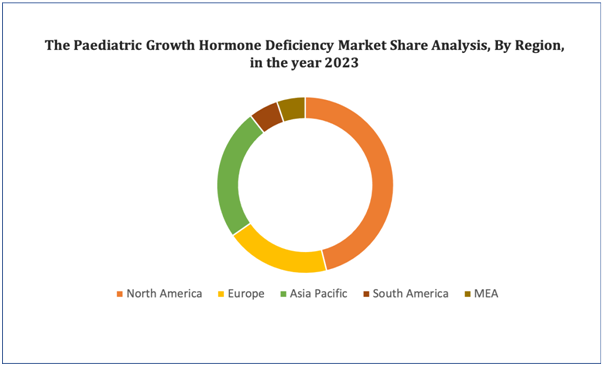
Get more details on this report - Request Free Sample
Leading Pediatric Growth Hormone Deficiency Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Pediatric Growth Hormone Deficiencymarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Pediatric Growth Hormone Deficiencymarket are briefly discussed below.
The report includes the list of players operating in the global Pediatric Growth Hormone Deficiencymarket. Some of the key players include:
- AnkeBio Co., Ltd. (China)
- Biopartners GmbH (Switzerland)
- Eli Lilly and Company (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Ferring B.V. (Switzerland)
- GeneScience Pharmaceuticals Co., Ltd. (China)
- Genentech, Inc. (U.S.)
- Ipsen Pharma (France)
- LG Chem (South Korea)
- Merck KGaA (Germany)
- Novartis AG (Switzerland)
- Novo Nordisk A/S (Denmark)
- Pfizer Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
Recent Developments in the Pediatric Growth Hormone DeficiencyMarket
Several recent developments have taken place in the field of pediatric growth hormone deficiency, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In June 2023, Pfizer Inc. and OPKO Health Inc. announced that the U.S. Food and Drug Administration (FDA) has approved NGENLA (somatrogon-ghla), a once-weekly, human growth hormone analog indicated for treatment of pediatric patients aged three years and older who have growth failure due to inadequate secretion of endogenous growth hormone. NGENLA is expected to become available for U.S. prescribing in August 2023.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
15.0% |
|
Type |
|
|
Treatment |
|
|
Diagnosis |
|
|
Symptoms |
|
|
Dosage |
|
|
Route of Administration |
|
|
End User |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
