Lyophilized Injectable Drugs Market Overview
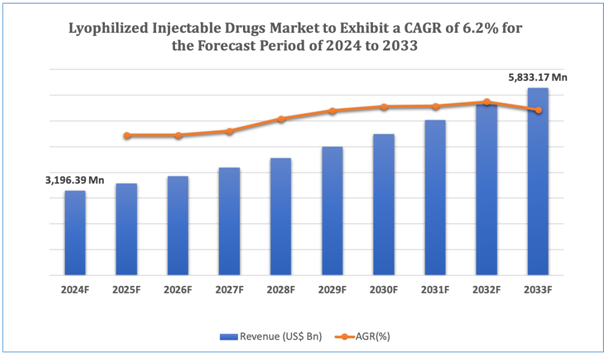
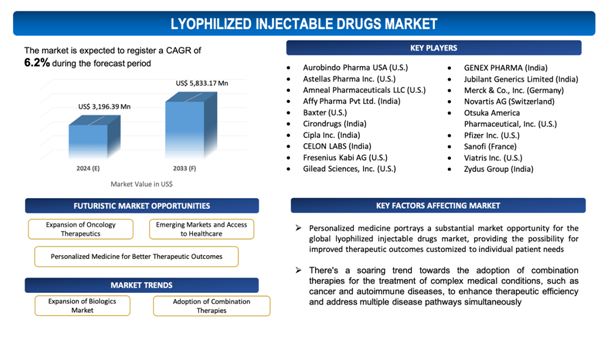
The global Lyophilized Injectable Drugs market is estimated to be worth over USD 5,833.17Mn in 2033 and is expected to grow at CAGR of6.2% during the forecast period (2024-2033).
Lyophilized injectable drugs, also commonly named as freeze-dried drugs, can be defined as pharmaceutical formulations that have been processed through lyophilization, a technique of dehydration that conserves the drug in a stable, solid state. This process includes freezing the drug solution and eradicating water under vacuum, led in a lyophilized cake that can be restored with a solvent, usually sterile water, before administration via injection. Lyophilized injectable drugs provide several advantages, including improved stability, extended shelf life, and reduced risk of degradation compared to liquid formulations. These drugs are commonly used for the treatment of various medical conditions, including infections, cancer, autoimmune diseases, and hormonal imbalances, among others.
The global market for lyophilized injectable drugs has witness substantial growth in the past few years, fuelled by several factors. The growingincidence of chronic diseases and the upsurge in demand for parenteral drug delivery methods have accelerated the adoption of lyophilized injectable drugs, particularly for therapeutics requiring long-term storage and stability. In addition to that, progressions in lyophilization technology and manufacturing processes have improved the efficacy and scalability of production, contributing to market expansion.
Along with that, the high soar of biopharmaceuticals and personalized medicine has further stimulated the demand for lyophilized injectable drugs, as they are well-suited for the formulation of proteins, peptides, monoclonal antibodies, and other complex biologics. Also, the globalization of pharmaceutical manufacturing and the expanding access to healthcare services in emerging markets have offers opportunities for market growth, as lyophilized injectable drugs offer logistical advantages such as ease of transportation, storage, and distribution.
As a whole, the global market for lyophilized injectable drugs is anticipated to witnesssustained expansion, propelled by factors such as growing disease burden, technological innovations, and evolving healthcare landscapes. With continuing research and development efforts focused at expanding the therapeutic applications of lyophilized injectable drugs and optimizing manufacturing processes, the market is estimated to witness further growth in the forthcoming years.
Figure 1. Lyophilized Injectable Drugs: Market Size
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global lyophilized injectable drugs market is marked by numerous key market insights and a dynamic panoramadefined by notable developments and significant technologies. Lyophilized injectable drugs continue to gain traction in the pharmaceutical industry owing to their inherent stability and extended shelf life, making them crucial for the treatment of various medical conditions. Significant developments in the market comprise the expansion of product portfolios to embody a comprehensivearray of therapeutic areas, comprising infectious diseases, oncology, autoimmune disorders, and hormone replacement therapy. Along with that, the market has experiencing a soaring focus on biologics and complex molecules, fuelling the demand for lyophilized formulations to sustain drug stability and efficiency.
Novel technologies have held an essential role in advancing the lyophilized injectable drugs market, with innovations emphasized at improving formulation processes, enhancing product quality, and maximizing manufacturing efficiency. Advanced lyophilization techniques, such as controlled nucleation and cycle optimization, have facilitated manufacturers to develop lyophilized drugs with superior characteristics, including reduced cycle times and improved reconstitution properties. Additionally, advancements in analytical methods and quality control measures have bolstered the development and regulatory approval of lyophilized injectable drugs, ensuring compliance with stringent safety and efficacy standards.
The current market panorama of the global lyophilized injectable drugs market is marked by a competitive environment, with leading players investing in research and development initiatives to innovate and diversify their product offerings. Collaborations and strategic partnerships between pharmaceutical firms and contract manufacturing organizations have also become prevalent, promoting access to specialized expertise and manufacturing capabilities. In addition to that, growing globalization and the soaring demand for biopharmaceuticals have encouraged investments in infrastructure and capacity expansion to meet increasing market demands. Overall, the global lyophilized injectable drugs market stands ready for continued growth, led by advancements in technology, expanding therapeutic applications, and evolving market dynamics.
Market Dynamics
Market Drivers
Increasing Geriatric Population with Chronic Illness
With aging comes anelevated susceptibility to chronic conditions such as diabetes, cancer, cardiovascular illnesses, and autoimmune disorders, requiringlong-drawn-out and efficient treatments. Lyophilized injectable drugs appear as a strategic solution, providing inherent benefitscustomized to the requirements of this demographic cohort. These formulations boast improved stability and extended shelf life, ensuring the preservation of drug potency over extended durations—a crucial factor in administering chronic ailments over the long term. Along with that, the convenience in administration and storage offered by lyophilized injectable drugs acknowledges the challenges related tocomplicated medication regimens usually encountered among elderly patients. This ease of use not only benefits patients but also aligns healthcare delivery for providers. In addition to that, the accurate dosing accuracy and high bioavailability associated with lyophilized formulations contribute to enhanced therapeutic outcomes, improving medication adherence and minimizing disease progression risks among aging populations. Since global demographics continue to skew older and chronic disease burdens amplify, the demand for lyophilized injectable drugs is estimated to riseat a significant pace. This projected growth trajectory underlines the crucial role of lyophilized injectable drugs in addressing the evolving healthcare needs of an aging population, positioning them as a foundation in the management of chronic illnesses across the world.
Market Restraints
With regard to numerous advantages of lyophilized injectable drugs, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- High Manufacturing Costs:The production of lyophilized injectable drugs encompassesintricate manufacturing processes, comprising freeze-drying and specialized equipment, leading to high production costs. The capital-intensive nature of lyophilization facilities, along with stringent regulatory requirements and quality control standards, further boils down to manufacturing expenses, constraining cost-effectiveness and profitability for manufacturers.
- Storage and Transportation Challenges:Lyophilized injectable drugs needstern temperature control and specialized storage conditions to maintain stability and efficiency, presenting logistical challenges in storage and transportation.The necessity for cold chain infrastructure and temperature-controlled shipping solutions surges operational costs and intricacy, particularly in regions with limited resources or inadequate infrastructure, hampering market penetration and accessibility of lyophilized injectable drugs.
Market Opportunities
Personalized Medicine for Better Therapeutic Outcomes
Personalized medicine portrays a substantial market opportunity for the global lyophilized injectable drugs market, providing the possibility for improved therapeutic outcomes customized to individual patient needs. Since healthcare transfers towards a more personalized approach, marked by the customization of treatment regimens based on patients' unique genetic makeup, medical history, and other factors, lyophilized injectable drugs emerge as an essential component in this paradigm. These formulations enable for precise dosing consistencyand accuracy, ensuring the delivery of optimal drug concentrations tailored to individual patient profiles. Additionally, lyophilized injectable drugs provide extended stability, conserving the integrity of the drug product during storage and administration, which is crucial for personalized medicine approaches needingparticular drug formulations. By enabling the development and delivery of personalized treatment alternatives, lyophilized injectable drugs empower healthcare providers to optimize therapeutic efficacy while minimizing adverse effects, ultimately improving patient outcomes. In addition to that, breakthroughs in lyophilization technology and manufacturing processes allow for the production of customized formulations with varying dosages, combinations, or release profiles, catering to the diverse needs of individual patients. As personalized medicine continues to gain impetus and adoption, accelerated by advancements in precision diagnostics and molecular profiling, the demand for lyophilized injectable drugs is expected to increase, propelling market growth and expansion. The potential of these formulations to promote personalized treatment strategies highlights their prominence in the evolving panorama of healthcare delivery, placing them as an essential tool in achieving better therapeutic results for patients across the globe.
Market Trends
- Expansion of Biologics Market:There's a perceptible trend towards the expansion of the biologics market, with an increasing number of therapeutic proteins, peptides, monoclonal antibodies, and other complex biologics being developed and commercialized.Lyophilized injectable drugs are well-suited for the formulation of biologics owing to their ability to maintain stability and integrity, making them a preferred alternative for drug manufacturers.With the rising demand for biologics led by advancements in biotechnology and growing prevalence of chronic ailments, the market for lyophilized injectable drugs is poised for significant growth as these formulations become increasingly crucial to the delivery of biologic therapies.
- Adoption of Combination Therapies:There's a soaring trend towards the adoption of combination therapies for the treatment of complex medical conditions, such as cancer and autoimmune diseases, to enhance therapeutic efficiency and address multiple disease pathways simultaneously.Lyophilized injectable drugs offer anall-around platform for formulating combination therapies, allowing for the co-administration of numerous drugs or biologics in a single dosage form.Since healthcare providers seek to optimize treatment outcomes and improve patient compliance, the demand for lyophilized injectable drugs capable of delivering combination therapies is anticipated to rise, fuelling market growth and innovation in formulation development.
Get more details on this report - Request Free Sample
Lyophilized Injectable Drugs Market: Key Segments
By Packaging
- Vials
- Dual-Chamber Syringes
- Dual-Chamber Cartridges
- Specialty Packaging
- Point-Of-Care Reconstitution
- Single-Use Vials
- Others
By Type of Delivery
- Single-Step Devices
- Multi-Step Devices
- Prefilled Diluent Syringes
- Proprietary Reconstitution Devices
By Drug Class
- Anti-Infective
- Antineoplastic
- Diuretics
- Proton Pump Inhibitor
- Anaesthetic
- Anticoagulant
- NSAID’s
- Corticosteroids
- Others
By Form
- Powder
- Liquid
By Indication
- Oncology
- Autoimmune Diseases
- Hormonal Disorders
- Metabolic Conditions
- Respiratory Diseases
- Gastrointestinal Disorders
- Dermatological Disorders
- Ophthalmic Diseases
- Infectious Diseases
- Others
By Route of Administration
- Intravenous/Infusion
- Intramuscular
- Others
By End User
- Hospital
- Clinics
- Ambulatory Surgery Centres (ASCs)
- Home Healthcare
- Others
By Distribution Channel
- Direct Tender
- Retail Sales
- Others
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Lyophilized Injectable Drugs Market: Regional Analysis
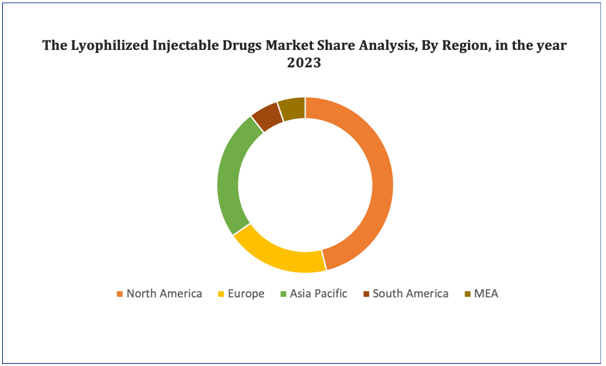
The North America, particular the U.S. is expected to lead the Lyophilized Injectable Drugs market, driven by a surge in research and developmental initiatives. With growing investment in innovative therapies and diagnostic advancements, the U.S. is positioned to offer cutting-edge treatments and interventions, solidifying its dominance in the regional market.In Europe, Germany leads the growth of the Lyophilized Injectable Drugs market due to expanding distribution channels. Leveraging a strong infrastructure, Germany efficiently disseminates innovative treatments and services, driving market expansion across the continent.In the Asia-Pacific region, China is dominates Lyophilized Injectable Drugs market's growth, propelled by increasing awareness of the condition and available therapies. With a burgeoning population and expanding healthcare infrastructure, China is driving advancements in Lyophilized Injectable Drugs treatment and support services, shaping market development in the region.
Figure 4. Lyophilized Injectable Drugs Market: Distribution by Region
Get more details on this report - Request Free Sample
Leading Lyophilized Injectable Drugs Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Lyophilized Injectable Drugsmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Lyophilized Injectable Drugsmarket are briefly discussed below.
The report includes the list of players operating in the global Lyophilized Injectable Drugsmarket. Some of the key players include:
- Aurobindo Pharma USA (U.S.)
- Astellas Pharma Inc. (U.S.)
- Amneal Pharmaceuticals LLC (U.S.)
- Affy Pharma Pvt Ltd. (India)
- Baxter (U.S.)
- Cirondrugs (India)
- Cipla Inc. (India)
- CELON LABS (India)
- Fresenius Kabi AG (U.S.)
- Gilead Sciences, Inc. (U.S.)
- GENEX PHARMA (India)
- Jubilant Generics Limited (India)
- Merck & Co., Inc. (Germany)
- Novartis AG (Switzerland)
- Otsuka America Pharmaceutical, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Viatris Inc. (U.S.)
- Zydus Group (India)
Recent Developments in the Lyophilized Injectable DrugsMarket
Several recent developments have taken place in the field of Lyophilized Injectable Drugs, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In September 2017, Cycle Pharmaceuticals Ltd announced that it has received approval from the U.S. Food and Drug Administration of its Abbreviated New Drug Application (“ANDA”) for Ketorolac Tromethamine Injection 30mg/mL. Ketorolac Tromethamine Injection is a nonsteroidal anti-inflammatory drug and is indicated for the short-term (up to 5 days in adults) management of moderately severe acute pain that requires analgesia at the opioid level.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
6.2% |
|
Packaging |
|
|
Type of Delivery |
|
|
Drug Class |
|
|
Form |
|
|
Indication |
|
|
Route of Administration |
|
|
End User |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
