Live Attenuated Vaccines Market Overview
The global Live Attenuated Vaccines market is estimated to be worth over USD 60,396.59 Mn in 2033 and is expected to grow at CAGR of12.3% during the forecast period (2024-2033).
Live attenuated vaccines can be perceived as a critical tool in global disease prevention efforts, leveringdebilitated forms of pathogens to encourage the immune system without inducing illness. These vaccines comprise live, but substantiallyimpaired, versions of the disease-inducing microorganism. By nearlyimitating natural infection, live attenuated vaccines provoking a strong immune response, offering long-lasting protection against specific diseases. Examples comprise the mumps, measles, and rubella (MMR) vaccine, oral polio vaccine (OPV), and the varicella (chickenpox) vaccine.
The global market for live attenuated vaccines is significant and continually evolving. Factors such as growing awareness of vaccination benefits, increasing initiatives for immunization programs in developing countries, and continuing research and development efforts fuelling market growth. In addition to that, the effectiveness and cost-effectiveness of live attenuated vaccines make them preferred choices for mass immunization campaigns. Market players, comprising pharmaceutical organizations, biotech firms, and governmental firms, continuously invest in developing and manufacturing live attenuated vaccines to address various infectious diseases.
Along with that, breakthroughs in vaccine delivery technologies, such as needle-free injection systems and thermostable formulations, enhance the accessibility and distribution of live attenuated vaccines, especially in resource-limited settings. However, restraints such as stringent regulatory requirements, concerns about vaccine safety, and the advent of vaccine hesitancy presentsbarriers to market expansion.
Regardless of these challenges, the global market for live attenuated vaccines is estimated to witness persistent growth in the forthcoming years. Continuing research into new vaccine candidates and expanded immunization efforts against existing diseases are expected to drive market demand. Moreover, collaborations between public and private sectors, along with initiatives like the World Health Organization's Expanded Program on Immunization (EPI), play pivotal roles in ensuring equitable access to live attenuated vaccines across the world. Since healthcare systems persist to prioritize disease prevention and control, live attenuated vaccines remain vital in protecting global public health.
Figure 1. Live Attenuated Vaccines: Market Size
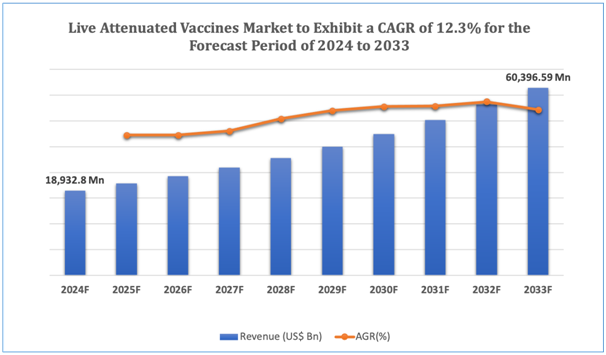
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global market for live attenuated vaccines can be portrayed by robust growth potential and dynamic market dynamics. Significant insights reveal a market fuelled by factors such as growingfocus on preventive healthcare, increasing prevalence of infectious diseases, and diversifying immunization programs across the globe. Crucial players in the market comprise leading pharmaceutical firms and biotechnology organizations, actively engaged in research, development, and commercialization of live attenuated vaccines targeting various infectious diseases.
Critical developments in the market have witnessed advancements in vaccine technology, comprising the development of novel delivery systems and adjuvants to improve vaccine efficacy and safety profiles. In addition to that, continuing research focuses on expanding the application of live attenuated vaccines to address emerging infectious diseases and unmet medical needs. Substantial advancements comprise the development of next-generation vaccines with enhanced stability, thermostability, and mitigated reactogenicity, supporting easier distribution and administration, specifically in resource-limited settings.
The current market landscape indicates a varied portfolio of live attenuated vaccines targeting a scale of infectious diseases, comprisingmumps, measles, polio,rubella, influenza, and varicella. These vaccines holdcrucial roles in routine immunization schedules, mass vaccination campaigns, and outbreak control efforts globally. Along with that, strategic collaborations between vaccine manufacturers, academic institutions, and governmental organizations focus onboosting vaccine development, improve production capacities, and encourage equitable access to vaccines across regions.
Alongside,conventional vaccine markets, emerging economies represent robust growth opportunities for live attenuated vaccines, fuelled by growing healthcare expenditure, expanding immunization infrastructure, and soaring awareness of vaccine-preventable diseases. However, challenges such as regulatory barriers, vaccine safety concerns, and supply chain limitations continue, requiringcollective efforts from stakeholders to address these barriers and foster market growth.
Moving ahead, the global live attenuated vaccines market stands ready for sustained expansion, propelled by continuous research and development initiatives, technological innovations, and collaborative partnerships focused at advancing vaccine science and enhancing global public health outcomes.
Market Dynamics
Market Drivers
Rising Prevalence of Infectious Diseases Worldwide
The surge in the prevalence of infectious diseases worldwide serves as a notable market driver for the global live attenuated vaccines market. Infectious diseases persist to pose as asignificant threat to public health, with factors such as urbanization, globalization, and climate change contributing to their spread and persistence. As a result, there is a growing demand for effective preventive measures, comprising vaccines, to restrain the transmission and impact of these diseases.
Live attenuated vaccines, which comprise weakened forms of pathogens, are specifically valuable in battling infectious diseases owing to their ability to imitate natural infections, paving its way to strong and long-lasting immune responses. They providenumerous benefits over other vaccine kinds, such as improved efficacy and fewer doses needed for immunization.
The soaring incidence of infectious diseases such as mumps,measles, rubella, influenza, and tuberculosis fuels the demand for live attenuated vaccines. For instance, measles outbreaks have surged in different regions, focusing on the necessity for vaccination campaigns and routine immunization programs. In the similar manner, the threat of emerging infectious diseases, exemplified by the COVID-19 pandemic, underlines the significance of preparedness and proactive vaccination strategies.
Along with that, government initiatives focused at expanding immunization coverage, along with investments in healthcare infrastructure and research and development, further accelerate market growth. These efforts emphasize on enhancing vaccine accessibility and affordability, particularly in low- and middle-income countries where infectious diseases disproportionately affect populations.
Furthermore, advancements in vaccine technologies and manufacturing processes contribute to the expansion of the live attenuated vaccines market, allowing the development of novel formulations and delivery methods to address evolving disease threats.
In conclusion, the increasing burden of infectious diseases across the world serves as a potent market driver for the global live attenuated vaccines market. With rising awareness of the importance of vaccination in disease prevention and control, coupled with supportive policies and technological innovations, the market stands ready for robust growth in the forthcoming future.
Market Restraints
With regard to numerous advantages of Live Attenuated Vaccines, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- Safety Concerns: Despite their effectiveness, live attenuated vaccines carry a risk of adverse reactions, particularly in individuals with weakened immune systems or specific medical conditions. Safety concerns regarding potential adverse effects, such as vaccine-related complications or the risk of reversion to virulence, is likely tohamper widespread acceptance and uptake of live attenuated vaccines.
- Storage and Transportation Challenges: Live attenuated vaccines often require stringent storage conditions, including specific temperature ranges and handling protocols, to maintain their potency and efficacy. The logistical challenges associated with cold chain management, especially in resource-limited settings or remote areas, can pose significant constraints on vaccine distribution, paving its way to wastage and limited accessibility for populations in need.
Market Opportunities
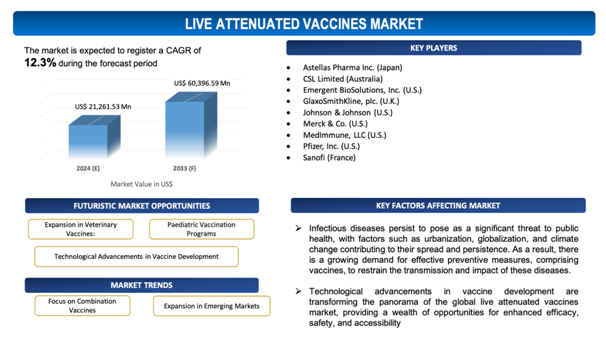
Technological Advancements in Vaccine Development
Technological advancements in vaccine development are transforming the panorama of the global live attenuated vaccines market, providing a wealth of opportunities for enhanced efficacy, safety, and accessibility. From sophisticated genetic engineering techniques to advanced biomanufacturing processes, these innovations are enabling the creation of vaccines that are not only more potent but also safer for widespread use. Furthermore, these technologies are expediting the vaccine development timeline, enabling for quicker responses to emerging infectious disease threats and public health crises. By harnessing the power of next-generation sequencing and computational modeling, researchers can tailor vaccine formulations to target specific strains or variants of pathogens, enhancing their effectiveness in diverse populations. Along with that, advancements in vaccine production techniques are increasing scalability and mitigating manufacturing costs, ultimately improving vaccine affordability and accessibility globally. Overall, technological innovation is fueling the live attenuated vaccines market forward, providing promising solutions to combat infectious diseases and improve global health outcomes.
Market Trends
- Focus on Combination Vaccines: One remarkable trend in the global live attenuated vaccines market is the soaring development and adoption of combination vaccines. These vaccines provide protection against multiple diseases in a single formulation, streamlining vaccination schedules and enhancing patient compliance. With the increasingfocus on vaccination coverage and disease prevention, pharmaceutical firms are investing in the research and development of combination vaccines, fuelling market growth and providingimproved convenience and efficiency to healthcare providers and patients alike.
- Expansion in Emerging Markets: Another prominent trend is the expanding presence of live attenuated vaccines in appearing markets. Swift urbanization, enhanced healthcare infrastructure, and government initiatives to expand immunization programs are fuellingsurging demand for vaccines against infectious diseases in regions such as Latin America, Asia-Pacific, and Africa. Pharmaceutical firms are capitalizing on this opportunity by diversifying their market reach, establishing partnerships with local healthcare providers, and investing in manufacturing facilities to cater to the growing demand for live attenuated vaccines in these regions. This trend is estimated to propel market growth and contribute to the collaborated efforts to eliminate or control infectious diseases.
Get more details on this report - Request Free Sample
Live Attenuated Vaccines Market: Key Segments
By Product Type
- Bacterial
- Viral
By Development
- Tissue Culture
- Embryonated Eggs
- Live Animals
By Indication
- Tuberculosis
- Measles
- Rotavirus
- Yellow Fever
- Oral Polio
- Others
By Mode of Administration
- Oral
- Injectable
- Others
By End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Live Attenuated Vaccines Market: Regional Analysis
North America is expected to dominate the market due to the increase in the expenditure for research and development proficiencies, increasing government initiatives, and improved healthcare infrastructure in various countries.Asia-Pacific is expected to exhibit the highest growth rate in the market during the forecast period due to the increasing government expenditure on the healthcare sector and rising technological advancements and initiatives by the government.
Figure 4. Live Attenuated Vaccines Market: Distribution by Region
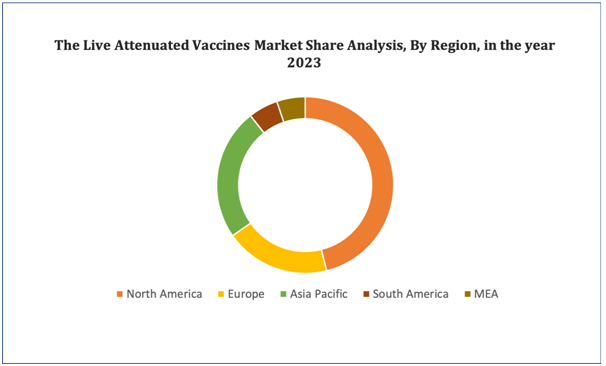
Get more details on this report - Request Free Sample
Leading Live Attenuated Vaccines Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Live Attenuated Vaccines market, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Live Attenuated Vaccines market are briefly discussed below.
The report includes the list of players operating in the global Live Attenuated Vaccines market. Some of the key players include:
- Astellas Pharma Inc. (Japan)
- CSL Limited (Australia)
- Emergent BioSolutions, Inc. (U.S.)
- GlaxoSmithKline, plc. (U.K.)
- Johnson & Johnson (U.S.)
- Merck & Co. (U.S.)
- MedImmune, LLC (U.S.)
- Pfizer, Inc. (U.S.)
- Sanofi (France)
- Serum Institute of India Pvt. Ltd. (India)
Recent Developments in the Live Attenuated Vaccines Market
Several recent developments have taken place in the field of Live Attenuated Vaccines, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In June 2021, KU Leuven developed a new, convenient and robust next-generation vaccine platform technology that allows to design and produce, at high yield, thermostable infectious vaccines. The technology was named PLLAV (Plasmid Launched Live Attenuated Virus) vaccine. In essence, the technology consists of a multi-host and easy-to-produce to high quantities Bacterial Artificial Chromosome (BAC) shuttle vector in which the genome of live-attenuated (flavi)virus vaccines (LAVs) can be cloned.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
12.3% |
|
Product Type |
|
|
Development |
|
|
Indication |
|
|
Mode of Administration |
|
|
End User |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
