Inflammatory Bowel Diseases (IBD) Market Overview
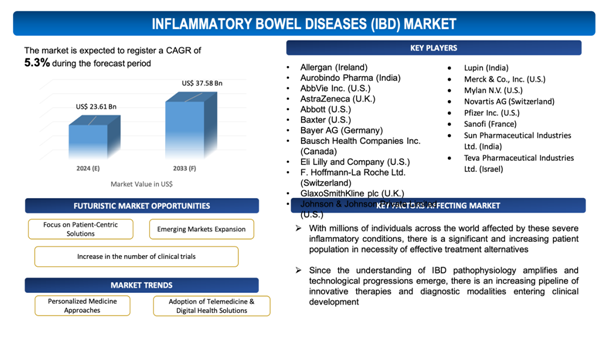
The global Inflammatory Bowel Diseases (IBD) market is estimated to be worth over USD 37.58 Bn in 2033 and is expected to grow at CAGR of5.3% during the forecast period (2024-2033).
Inflammatory Bowel Diseases (IBD) portray a class of chronic inflammatory conditions affecting the gastrointestinal tract, especially comprising Crohn's disease and Ulcerative Colitis. These conditions be associated from an abnormal immune response against the intestinal microbiota in genetically susceptible individuals, which may lead to chronic inflammation and tissue damage. IBD generally manifests with symptoms such as diarrhoea, abdominal pain, weight loss, rectal bleeding, and fatigue, primarilyharming patients' quality of life. The exact etiology of IBD remains evasive, although factors like environmental triggers, genetic predisposition, gut microbiota dysbiosis, and immune system dysfunction are believed to play critical roles in disease development.
The global market for IBD therapeutics and diagnostics has experiencedsignificant growth attributing to the growing prevalence and incidence rates of IBD across the world. Factors such as enhanced disease awareness, improved diagnostic techniques, and progressions in treatment alternatives have also contributed to market expansion. Biologic agents, corticosteroids, immunosuppressants, amino salicylates, and antibiotics are among the mainstay treatments for IBD, emphasizingon inducing and maintaining remission while alleviating symptoms and mitigating inflammation-associated complications. Biologic therapies, specifically anti-TNF agents like infliximab and adalimumab, have transformed IBD management, providing effective targeted therapy for patients with moderate to severe disease who are refractory to traditional treatments.
Along with that, the IBD market is experiencing a rise in research and development activities, with pharmaceutical firms focusing on novel therapeutic approaches such as biosimilars, small molecule inhibitors, and personalized medicine strategies. In addition, advancements in diagnostic tools, comprisingbiomarker identification, genetic testing, endoscopic imaging techniques, and non-invasive monitoring modalities, are improving disease detection, prognosis, and treatment optimization.
However, regardless of these advancements, challenges continue in the IBD market, comprising high treatment costs, limited accessibility to advanced therapies in certain regions, and the risk of adverse effects related to immunosuppressive agents. Along with that, the complicated and heterogeneous nature of IBD poses challenges in disease management and needs a multidisciplinary approach involving immunologists, gastroenterologists, nutritionists,surgeons, and psychologists.
Thus, inflammatory bowel diseases represent a substantial global health burden, requiringcontinuous efforts to advance therapeutic and diagnostic modalities. The expanding IBD market offers opportunities for innovation and investment aimed at improving patient outcomes and addressing unmet medical needs in this chronic and exhaustive condition.
Figure 1. Inflammatory Bowel Diseases (IBD): Market Size

Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global market for Inflammatory Bowel Diseases (IBD) is witnessingremarkable growth and evolution led by numerous key market insights and developments. With the growing prevalence of IBD across the globe, estimated at over 3 million cases in the United States alone, there is a soaring demand for advanced therapeutics and diagnostics to tackle the intricate nature of the disease. Biologic agents, specifically anti-TNF drugs like infliximab and adalimumab, dominate the current market panorama, providing effective targeted therapy for moderate to serious IBD cases. However, the market is experiencing a move towards novel treatment modalities such as small molecule inhibitors, biosimilars, and personalized medicine approaches, indicating the necessity for enhanced safety, efficiency, and patient-specific treatment strategies.
In addition to that, substantial advancements in diagnostic technologies are redefining the IBD market panorama, allowing more precise disease detection, prognosis, and treatment monitoring. Biomarker identification, genetic testing, and advanced endoscopic imaging techniques are among the major developments improving clinicians' ability to customize treatment strategies on the basis of individual patient characteristics and disease phenotypes. Non-invasive monitoring modalities such as fecal calprotectin testing and imaging modalities like magnetic resonance enterography are also gaining traction, offering valuable insights into disease activity and progression without the need for invasive procedures.
Along with that, the market is experiencinggrowing investment in research and development activities focused at determining novel therapeutic targets and innovative treatment approaches. With a soaring emphasis on precision medicine and personalized therapies, the IBD market stands ready for further expansion and innovation, fueled by collaborations between pharmaceutical companies, academic institutions, and healthcare providers to address the unmet needs of patients with IBD.
Market Dynamics
Market Drivers
High Prevalence Rate of Crohn's Disease and Ulcerative Colitis
With millions of individuals across the world affected by these severe inflammatory conditions, there is a significant and increasing patient population in necessity of effective treatment alternatives. Each year, more or less 70,000 new instances of inflammatory bowel disease are diagnosed in the United States, as per the Crohn's and Colitis Foundation (CCFA). In addition, according to a 2019 CCFA estimate, approximately 1.6 million people in the United States suffered from IBD. In parallel, the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) estimates that 3.4 million people in Europe was living with IBD in 2020.This prevalence translates into a large addressable market for pharmaceutical organizations and medical device manufacturers, incentivizing investment in research and development activities to advancenovel therapies and diagnostic tools. Furthermore, the chronic nature of Crohn's disease and ulcerative colitis needs long-term management, establishing a continuous demand for maintenance therapies and monitoring solutions. As a consequence, the increased prevalence rate not only surges the market size but also fuels competition among industry players, which may lead to advancements in treatment alternatives, enhanced patient outcomes, and improved overall care for individuals living with IBD.
Market Restraints
With regard to numerous advantages of Inflammatory Bowel Diseases (IBD), the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- High Treatment Costs: The cost of IBD therapeutics, specifically biologic agents and novel therapies, can be unusually expensive, presenting a financial strain on patients and healthcare systems. Limited insurance coverage and out-of-pocket expenses further aggravate affordability issues, limiting access to advanced treatments for some individuals with IBD.
- Adverse Effects of Immunomodulatory Therapies: Whilst immunosuppressive agents and biologic therapies are effective in administering IBD, they also carry the risk of side effects such as greater susceptibility to infections, infusion reactions, and long-term complications like malignancies. Concerns regarding safety profiles are likely toprevent patients and healthcare providers from initiating or continuing treatment with these agents, impacting market uptake and patient adherence
Market Opportunities
Increase in the Number of Clinical Trials
Clinical trials serve as crucial platforms for assessing the efficiencyand safety of new diagnostic tools, therapeutic interventions, and treatment approaches for IBD. Since the understanding of IBD pathophysiology amplifies and technological progressions emerge, there is anincreasing pipeline of innovative therapies and diagnostic modalities entering clinical development.According to the US National Library of Medicine, there are 730 clinical trials (including medication development, observational studies, and others) connected to IBD as of October 2019, with 268 clinical trials being undertaken just in the United States.
These trials provide the potential to address unmet medical needs, improve treatment outcomes, and expand the therapeutic armamentarium available to healthcare providers and patients. In addition to that, the diversification of clinical trial designs, comprising randomized controlled trials, observational studies, and real-world evidence analyses, promotes comprehensive assessment across various disease severities, patient populations, and therapeutic strategies.
What is more, the shared nature of clinical research, including partnerships between pharmaceutical firms, academic institutions, contract research organizations, and regulatory agencies, fosters innovation and propels the translation of scientific discoveries into clinical practice. In the end, the propogation of clinical trials in the IBD space not only fuels advancements in disease management but also encourages economic growth, investment opportunities, and market expansion within the global IBD market biome.
Market Trends
- Personalized Medicine Approaches: With progressions in precision medicine and biomarker identification, there is a soaring trend towards personalized treatment strategies customized to individual patient characteristics, disease phenotypes, and treatment responses. By leveraging immunological profiling, genetic testing, and other molecular diagnostics, healthcare providers can optimize therapeutic decision-making, improve treatment efficiency, and mitigate adverse effects, fuelling the adoption of targeted therapies and personalized care paradigms in the management of IBD.
- Adoption of Telemedicine and Digital Health Solutions: The COVID-19 pandemic has propelled the adoption of telemedicine and digital health solutions in healthcare delivery, comprising the management of chronic conditions like IBD. Telemedicine platforms, remote monitoring tools, and mobile health applications allow virtual consultations, remote patient monitoring, and real-time data collection, supporting continuous disease management, improving patient engagement, and enhancing access to specialized care for individuals with IBD. This trend towards digital health integration is estimated to continue post-pandemic, defining the future of IBD care delivery and spurring innovation in remote patient management strategies.
Get more details on this report - Request Free Sample
Inflammatory Bowel Diseases (IBD) Market: Key Segments
By Type
- Microscopic Colitis
- Ulcerative Colitis
- Crohn's Disease
By Treatment
- Medication
- Corticosteroids
- Biologics
- Immunosuppressant
- Anti-diarrheal Medications
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Antibiotics
- Others
- Surgery
- Others
By Diagnosis
- Imaging Procedures
- Magnetic resonance imaging (MRI)
- Computerized tomography (CT) scan
- X-ray
- Endoscopic Procedures
- Balloon-assisted enteroscopy
- Capsule endoscopy
- Upper endoscopy
- Flexible sigmoidoscopy
- Colonoscopy
- Lab Tests
- Stool studies
- Tests for anemia or infection
By Dosage Form
- Tablet
- Capsule
- Injections
- Others
By Route of Administration
- Oral
- Parenteral
- Others
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Inflammatory Bowel Diseases (IBD) Market: Regional Analysis
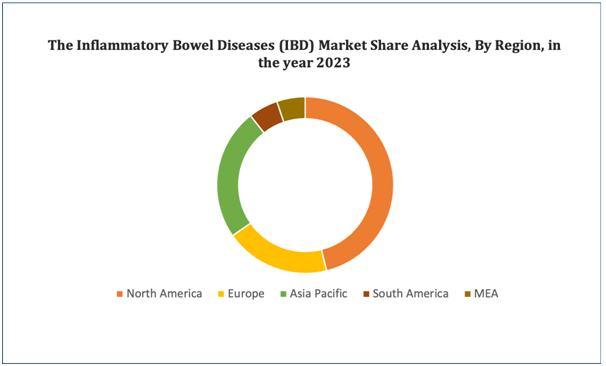
North America dominates the inflammatory bowel diseases market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the growing demand for a specific treatment, and rising healthcare expenditure will further propel the market's growth rate in this region. Additionally, presence of major key players and high prevalence of inflammatory bowel diseases, including ulcerative colitis and Crohn's disease. will further propel the market's growth rate in this region.Asia-Pacific is expected to be the fastest-growing region during the forecast period due to growing level of disposable income in this region. Also, the development of healthcare infrastructure and rising government initiatives will further propel the market's growth rate in this region.
Figure 4. Inflammatory Bowel Diseases (IBD) Market: Distribution by Region
Get more details on this report - Request Free Sample
Leading Inflammatory Bowel Diseases (IBD) Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Inflammatory Bowel Diseases (IBD)market, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Inflammatory Bowel Diseases (IBD)market are briefly discussed below.
The report includes the list of players operating in the global Inflammatory Bowel Diseases (IBD)market. Some of the key players include:
- Allergan (Ireland)
- Aurobindo Pharma (India)
- AbbVie Inc. (U.S.)
- AstraZeneca (U.K.)
- Abbott (U.S.)
- Baxter (U.S.)
- Bayer AG (Germany)
- Bausch Health Companies Inc. (Canada)
- Eli Lilly and Company (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- GlaxoSmithKline plc (U.K.)
- Johnson & Johnson Private Limited (U.S.)
- Lupin (India)
- Merck & Co., Inc. (U.S.)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Sun Pharmaceutical Industries Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
Recent Developments in the Inflammatory Bowel Diseases (IBD)Market
Several recent developments have taken place in the field of Inflammatory Bowel Diseases (IBD), some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In October 2023,Sanofi and Teva Pharmaceuticals, a U.S. subsidiary of Teva Pharmaceutical Industries Ltd. announce a collaboration to co-develop and co-commercialize asset TEV’574, currently in Phase 2b clinical trials for the treatment of Ulcerative Colitis and Crohn's Disease, two types of inflammatory bowel disease.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
5.3% |
|
Type |
|
|
Treatment |
|
|
Diagnosis |
|
|
Dosage Form |
|
|
Route of Administration |
|
|
End User |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
