Immunotherapy Drugs Market Overview
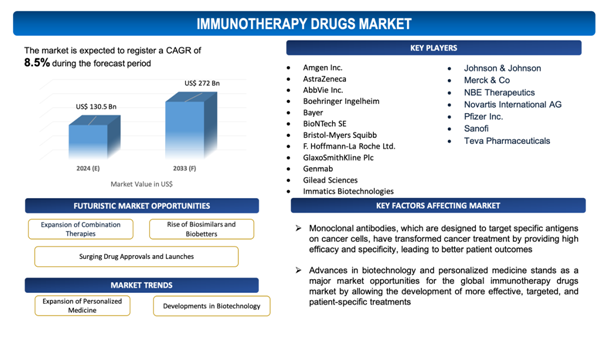
The global immunotherapy drugs market is estimated to be worth over USD 272 Bnin 2033 and is expected to grow at CAGR of 8.5% during the forecast period (2024-2033).
Immunotherapy drugs are transforming the healthcare panorama by leveraging the body's immune system to combat diseases, characterizing a substantial departure from conventional treatments that immediately target disease cells. This advanced approach comprises different therapies such as CAR T-cell therapy, checkpoint inhibitors, monoclonal antibodies, and cancer vaccines, each holding a critical role in improving the immune response against cancer and other diseases.
Checkpoint inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo) work by blocking proteins that hinder immune cells from attacking cancer cells, illustrating substantial success in managing melanoma, lung cancer, and renal cancer. CAR T-cell therapy comprises engineering a patient’s T-cells to target and destroy cancer cells, exhibiting noteworthy results in blood cancers like leukemia and lymphoma. Monoclonal antibodies, intended to bind to specific antigens on cancer cells, aid the immune system determine and eradicate these cells. Cancer vaccines encourage the immune system to battle cancer cells, providing a proactive approach to cancer prevention and treatment.
The impact of immunotherapy prolongs beyond oncology, exhibiting commitment in treating autoimmune diseases, infectious diseases, and allergies. These therapies provide personalized treatment alternatives, enhancing survival rates and quality of life for patients who previously had limited alternatives. The potential for lesser side effects in contrast to conventional treatments like chemotherapy and radiation is a notable benefit, making immunotherapy an appealingchoice for many patients.
In addition to that, continuous research and clinical trials are expanding the applications of immunotherapy, exploring its effectiveness in a wider range of diseases and patient populations. As a consequence, immunotherapy is not only revolutionizing the way we approach disease treatment but also leading the way for more effective, precise, and less toxic therapeutic alternatives. This paradigm movehighlights the essential role of the immune system in disease management and underscores the potential of immunotherapy to reshape the future of medicine.
Figure 1.Immunotherapy Drugs: Market Size
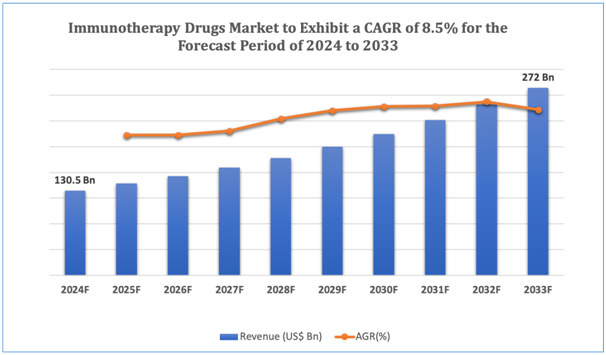
Get more details on this report - Request Free Sample
Key Market Insights
The immunotherapy drugs market is witnessingstrong growth, fuelled by substantialdevelopments and a growing emphasis on personalized medicine. Valued at over $120 billion in 2023, it is anticipated to expand swiftlyowing to increasing cancer prevalence, escalated approvals of novel therapies, and progressions in biotechnology. Significant developments comprise the approval of new checkpoint inhibitors and CAR T-cell therapies, which have illustrated high efficacy in treating different cancers. Notable checkpoint inhibitors like pembrolizumab and nivolumab have been expanded to treat numerous cancer types, while CAR T-cell therapies such as Kymriah and Yescarta are making progress in hematologic malignancies.
Advanced technologies are also defining the market, with developments in gene editing tools like CRISPR improving the effectiveness and precision of immunotherapies. Bispecific antibodies, designed to engage two different targets simultaneously, are evolving as a strong tool in the battle against cancer. In addition, personalized neoantigen vaccines, which are personalized to individual patients’ tumour profiles, are exhibitingcommitment in clinical trials.
The competitive panorama is characterized by substantial investment from pharmaceutical giants such as Merck, and Novartis, alongside major biotech firms. Collaborations and partnerships are common, focused at propelling the development of next-generation immunotherapies. Regardless of high costs, the growing body of evidence facilitating the long-term benefits of immunotherapies is fostering broader adoption. As regulatory agencies continue to approve new therapies and indications, the immunotherapy market stands ready for sustained growth, essentiallyrevolutionizing cancer treatment and providing new hope for patients globally.
Market Dynamics
Market Drivers
Growing Demand for Monoclonal Antibodies and Biosimilars
Monoclonal antibodies, which are designed to target specific antigens on cancer cells, have transformed cancer treatment by providing high efficacy and specificity, leading tobetter patient outcomes. Their capability to accurately target diseased cells while sparing healthy ones minimizes side effects in contrary toconventional therapies like chemotherapy, making them highly attractive to healthcare providers and patients alike. The success of monoclonal antibodies in treating different cancers, autoimmune diseases, and infectious diseases has accelerated their demand, resulting insignificant market expansion.
Biosimilars, which are near-identical copies of existing biologic drugs, are also accelerating market growth. As patents on severalsuccessful monoclonal antibodies expire, biosimilarsoffer a cost-effective alternative, improving accessibility and affordability. This is specifically impactful in developing markets, where cost restrictions have historically bounded access to advanced biologics. The entry of biosimilars fosters competitive pricing, further fuelling market penetration and adoption. Regulatory frameworks in major regions like the US and Europe have evolved to streamline the approval process for biosimilars, ensuring their safety and efficacy while expediting their market entry.
Along with that, the persistent innovation and development of novel monoclonal antibodies customized for specific targets and the increasing investment in research and development are expanding the therapeutic applications of these drugs. Together, the growing demand for monoclonal antibodies and the escalation of biosimilars are leading factors fuelling the robust growth of the global immunotherapy drugs market, making cutting-edge treatments more accessible to a wider patient population.
Market Restraints
With regard to numerous advantages of Immunotherapy Drugs, the market faces several challenges due to the unique characteristics and requirements associated with these potent pharmaceutical products. Some of the key market challenges include:
- High Treatment Costs: Immunotherapy drugs, specifically novel treatments such as CAR T-cell therapy and checkpoint inhibitors, are time and again expensive, posing a major financial strain on patients and healthcare systems. The increased costs can impede affordability and accessibility, particularly in developing regions, thus restraining market growth. Even with the emergence of biosimilars, which provide more cost-effective alternatives, the overall expense related to immunotherapy remains a significant barrier.
- Complex Manufacturing and Regulatory Challenges: The production of immunotherapy drugs, specifically personalized treatments like CAR T-cell therapy, comprises complex and time-consuming processes that need specialized facilities and expertise. This complexity can result in supply chain challenges and delays in drug availability. In addition, stringent regulatory requirements for approval and quality control add further barriers, making it complex for new therapies to quickly reach the market. These factors collectively act as restraints, decelerating the expansion of the global immunotherapy drugs market.
Get more details on this report - Request Free Sample
Market Opportunity
Developments in Biotechnology and Personalized Medicine
Advances in biotechnology and personalized medicine stands as amajor market opportunities for the global immunotherapy drugs market by allowing the development of more effective, targeted, and patient-specific treatments. Leading-edge biotechnological innovations, such as next-generation sequencing, CRISPR gene editing, and advanced biomanufacturing techniques, promote the creation of sophisticated immunotherapies that can accurately target cancer cells and other disease-inducing agents with minimal off-target effects. Personalized medicine, which tailors treatments to an individual’s genetic profile, is specificallyrevolutionaryfor immunotherapy. By understanding the unique genetic and molecular makeup of a patient’s disease, clinicians can design highly specific immunotherapies that improve efficacy and minimizeside effects.
This personalized approach is demonstrated by the development of neoantigen vaccines and CAR T-cell therapies customized to each patient’s tumour profile. Such treatments have shown remarkable success in clinical trials, substantiallyenhancing patient outcomes and survival rates. In addition, biotechnology advancements are propelling the discovery and development of novel biomarkers, which can foretell patient response to immunotherapy, enabling for more effective and precise treatment regimens.
The convergence of biotechnology and personalized medicine also accelerates the drug development process, reducing time and costs associated with bringing new therapies to market. This improves the ability of pharmaceutical companies to meet the growing demand for advanced immunotherapies. As these technologies continue to evolve, they expand the therapeutic potential of immunotherapies beyond oncology, opening new avenues in treating autoimmune diseases, infectious diseases, and other chronic conditions. Therefore, the alliance between biotechnology and personalized medicine portrays a powerful opportunity to drive the growth and innovation of the global immunotherapy drugs market.
Market Trends
- Expansion of Combination Therapies: There is a growing trend toward developing and utilizing combination therapies in the immunotherapy drugs market. Combining different types of immunotherapies, such as checkpoint inhibitors with CAR T-cell therapy or monoclonal antibodies with cancer vaccines, aims to enhance overall treatment efficacy and overcome resistance mechanisms. This approach is showing promising results in clinical trials, leading to improved patient outcomes and expanding the therapeutic applications of immunotherapies across various cancer types and other diseases.
- Rise of Biosimilars and Biobetters: The market is experiencing a soaring introduction and adoption of biosimilars and biobetters as patents on original biologic drugs expire. Biosimilars are near-identical copies of approved biologic drugs, offering similar efficacy and safety profiles at lower costs, thusenhancing accessibility and affordability. Biobetters, on the other hand, are modified versions of existing biologics with improved properties, such as improved efficacy, minimizedadverse effects, or more convenient administration methods. This trend not only fosters competitive pricing but also fuels innovation, as pharmaceutical companies strive to distinguish their products in a congested market.
Immunotherapy Drugs Market: Key Segments
By Type
- Monoclonal Antibodies
- Checkpoint Inhibitors
- Interferons&Interleukins
- Other Immunotherapies
By Therapy Area
- Cancer
- Autoimmune &Inflammatory Diseases
- Infectious Diseases
- Other Therapeutic Areas
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Figure 4. Immunotherapy Drugs Market: Distribution by Region
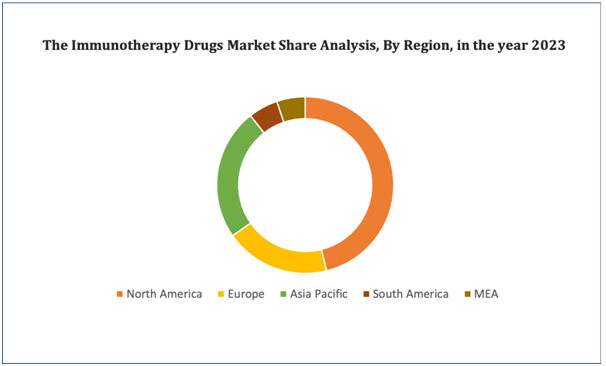
Get more details on this report - Request Free Sample
Immunotherapy Drugs Market: Regional Analysis
North America accounted for the largest share of the global market. The large share of this region in the global market can be attributed to the increasing prevalence of cancer and autoimmune diseases, rising demand for safer cancer therapies, increasing number of approvals from the FDA, and the introduction of favorable reimbursement policies. The Asia Pacific market is expected to grow at the highest rate during the forecast period.
Leading Immunotherapy Drugs Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the immunotherapy drugsmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the immunotherapy drugsmarket are briefly discussed below.
The report includes the list of players operating in the global immunotherapy drugs market. Some of the key players include:
- Amgen Inc.
- AstraZeneca
- AbbVie Inc.
- BoehringerIngelheim
- Bayer
- BioNTech SE
- Bristol-Myers Squibb
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline Plc
- Genmab
- Gilead Sciences
- Immatics Biotechnologies
- Johnson & Johnson
- Merck &Co
- NBE Therapeutics
- Novartis International AG
- Pfizer Inc.
- Sanofi
- Teva Pharmaceuticals
Immunotherapy Drugs Market: Key Developments
- In March 2024, Bristol Myers Squibb announced the U.S. Food and Drug Administration (FDA) has granted accelerated approval of Breyanzi ® (lisocabtagenemaraleucel; liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received at least two prior lines of therapy, including a Bruton tyrosine kinase (BTK) inhibitor and a B-cell lymphoma 2 (BCL-2) inhibitor. This indication is approved under accelerated approval based on response rate and duration of response.
- In January 2023, the Food and Drug Administration (FDA) approved pembrolizumab (Keytruda, Merck) for adjuvant treatment following resection and platinum-based chemotherapy for stage IB (T2a ≥4 cm), II, or IIIA non-small cell lung cancer (NSCLC).
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
|
Key Report Attributes |
Details |
|
|
|
Base Year |
2023 |
|
|
|
Forecast Period |
2024-2033 |
|
|
|
CAGR (2024-2033) |
8.5% |
|
|
|
Type |
|
|
|
|
Therapy Area |
|
|
|
|
Distribution Channel |
|
|
|
|
Key Geographical Regions |
|
|
|
Key Companies Profiled |
|
|
|
