Hepatitis C Market Overview
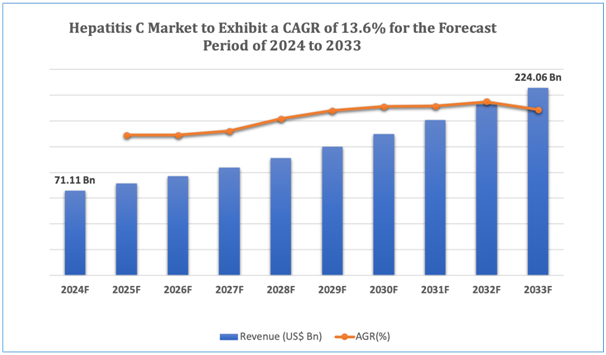
The global Hepatitis C market is estimated to be worth over USD 224.06Bn in 2033 and is expected to grow at CAGR of 13.6% during the forecast period (2024-2033). Hepatitis C represents a viral infection caused by the hepatitis C virus (HCV) and is a critical global health concern. An approximate 71 million people across the world are living with chronic HCV infection. The virus principally affects the liver and can resultinserious liver-associated complications such as liver failure, cirrhosis, and hepatocellular carcinoma, making it a primary cause of liver-associated morbidity and mortality globally. Hepatitis C is essentially transmitted through exposure to infected blood, most generally through injecting drug use, unsafe medical practices, and transfusion of unscreened blood and blood products.
The global market for hepatitis C surroundsdifferent sectors emphasized at acknowledging the burden of HCV infection and its related complications. Diagnostics hold a crucial role in the early detection and monitoring of hepatitis C, with tests such as HCV antibody screening and HCV RNA quantification supporting in diagnosis and disease management. Therapeutic interventions for hepatitis C have experienced a revolution in the past years with the development of direct-acting antiviral medications. These medications target specific viral proteins and hinder viral replication, resulting in high cure rates exceeding 95% and substantiallyenhancing patient outcomes.
Regardless of these advancements, challenges remain in achieving widespread access to hepatitis C testing, treatment, and prevention, specifically in low- and middle-income countries where resources are likely to be limited. Addressing these challenges necessitates concerted efforts from governments, healthcare organizations, industry stakeholders, and advocacy groups to implement comprehensive hepatitis C control programs, increase public awareness, and ensure equitable access to care for all individuals affected by HCV infection. Continued research, innovation, and collaboration are prominent to further advance the global response to hepatitis C and mitigate its impact on global public health.
Figure 1. Hepatitis C: Market Size
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global hepatitis C market is marked by notable advancements and a dynamic panoramafueled by advanced therapies and developed diagnostic technologies. Significant developments comprise the introduction of increasingly effective direct-acting antiviral medications, which have transformed hepatitis C treatment by providing cure rates exceeding 95% and substantiallyenhancing patient outcomes. These medications target specific viral proteins, impeding viral replication and paving its way to continued virologic response in the majority of treated patients. In addition to that, novel diagnostic technologies, such as HCV RNA quantification assays and point-of-care testing devices, allow accurate diagnosis and monitoring of hepatitis C, supporting timely treatment initiation and disease management. The market outlook is further influenced by efforts to improve access to hepatitis C testing and treatment, especially in low- and middle-income countries where the burden of HCV infection is high. Persistent research and development efforts are emphasized on developing next-generation antiviral therapies with enhancedefficiency, safety profiles, and ease of administration, as well as expanding access to care through innovative healthcare delivery models and strategic partnerships.
Market Dynamics
Market Drivers
Increasing Research and Development Efforts
The growing prevalence of hepatitis is a primary factor fueling the hepatitis C market's growth rate. As per the World Health Organization (WHO) data published in July 2021, an approximate 58 million individuals have chronic hepatitis C virus infection, with 1.5 million new infections occurring every year. The hepatitis C virus can be perceived as a blood-borne pathogen, and the most prevalent way to contract it is by contact with small amounts of blood. This frequently occurs as a consequence of improper injections and poor health care.
Since awareness of hepatitis C increases and screening efforts grows, more individuals are being diagnosed with the virus, fueling demand for hepatitis C testing and treatment services. In addition to that, the increased prevalence of HCV infection contributes to the overall economic burden on healthcare systems, paving its way to increased investment in research, development, and innovation in the hepatitis C market. Pharmaceutical firms are incentivized to develop new therapies and diagnostics to meet the growing demand for effective hepatitis C management options. Along with that, the growing prevalence of hepatitis C presents an opportunity for healthcare providers and policymakers to prioritize hepatitis C control efforts, implement comprehensive prevention programs, and diversify access to care, ultimately enhancing outcomes for individuals affected by this viral infection.
Market Restraints
With regard to numerous advantages of Hepatitis C, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- Cost of Treatment: The increased cost of direct-acting antiviral medications for hepatitis C treatment can act as a leading barrier to access, specifically in low- and middle-income countries and for patients without adequate insurance coverage, limiting affordability and availability of treatment alternatives
- Stigma and Awareness: Stigma surrounding hepatitis C and lesser awareness about the virus among both the general population and healthcare providers can impede efforts to scale up screening, diagnosis, and treatment initiatives, resultingin underdiagnosis and undertreatment of individuals with HCV infection.
Market Opportunities
Launch of New Products
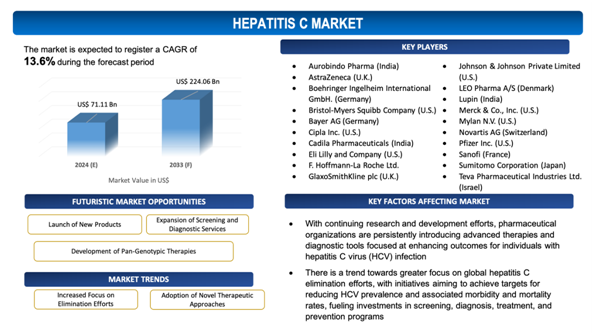
With continuing research and development efforts, pharmaceutical organizations are persistently introducing advanced therapies and diagnostic tools focused at enhancing outcomes for individuals with hepatitis C virus (HCV) infection. Mavyret (glecaprevir and pibrentasvir) pills, for instance, were approved by the US Food and Drug Administration in April 2019 to treat six genotypes of hepatitis C virus (HCV) in children aged 12 to 17. Furthermore, in June 2021, Gilead Sciences, Inc. got FDA approval for Epclusa for the treatment of chronic hepatitis C virus (HCV) in children under the age of three, regardless of HCV genotype or severity of liver disease.The development of new direct-acting antiviral medications with improvedefficiency, safety profiles, and resistance barriers provides commitment for achieving increased cure rates and shorter treatment durations, ultimately enhancing patient adherence and satisfaction. In addition to that, novel diagnostic technologies facilitate more accurate and timely detection of HCV infection, facilitating early intervention and treatment initiation. The introduction of these new products not only diversifies treatment alternatives for patients but also encourages competition in the market, potentially playing down drug prices and enhancing affordability and accessibility of hepatitis C care. Along with that, the launch of new products fosters innovation and collaboration within the healthcare industry, fueling advancements in therapeutic approaches, diagnostic techniques, and healthcare delivery models aimed at addressing the evolving needs of individuals affected by hepatitis C across the globe.
Market Trends
- Increased Focus on Elimination Efforts: There is a trend towards greater focus on global hepatitis C elimination efforts, with initiatives aiming to achieve targets for reducing HCV prevalence and associated morbidity and mortality rates, fueling investments in screening, diagnosis, treatment, and prevention programs.
- Adoption of Novel Therapeutic Approaches: There is a trend towards the adoption of novel therapeutic approaches in the treatment of hepatitis C, comprising combination therapies, immune modulators, and host-targeting agents, as well as the development of pan-genotypic antiviral medications, improving treatment efficiency and addressing challenges such as drug resistance and genotype diversity.
Get more details on this report - Request Free Sample
Hepatitis C Market: Key Segments
By Type
- Acute Hepatitis C
- Chronic Hepatitis C
- Other
By Treatment
- Antiviral Drugs
- Telbivudine
- Entecavir
- Tenofovir disoproxil
- Lamivudine
- Others
- Vaccine
- Immune Modulator Drugs
- Pegylated interferon
- Interferon alpha
- Surgery
- Others
By Diagnosis
- Liver Biopsy
- Blood Tests
- Imaging Tests
- Ultrasound
- MRI
- CT scan
- Others
By Route of Administration
- Oral
- Parenteral
- Others
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Hepatitis C Market: Regional Analysis
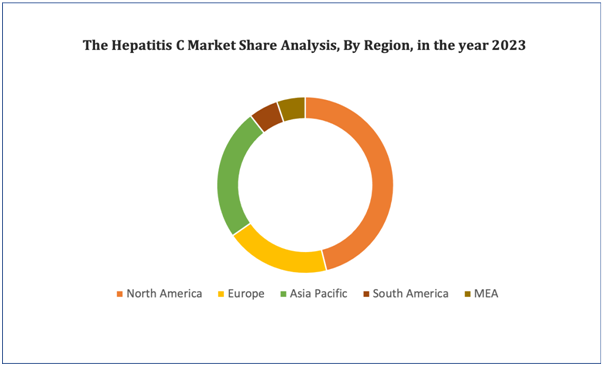
North America dominates the hepatitis C market because of the growing presence of major key players and the rising number of research and development activities will further propel the market’s growth rate in this region. Additionally, an increase in number of FDA approval drugs will further propel the market’s growth rate in this region.Asia-Pacific are expected to grow during the forecast period due to surging number of generic manufacturers and growing government initiatives in this region. Also, development of healthcare infrastructure will further propel the market’s growth rate in this region.
Figure 4. Hepatitis C Market: Distribution by Region
Get more details on this report - Request Free Sample
Leading Hepatitis C Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Hepatitis Cmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Hepatitis Cmarket are briefly discussed below.
The report includes the list of players operating in the global Hepatitis Cmarket. Some of the key players include:
- Aurobindo Pharma (India)
- AstraZeneca (U.K.)
- Boehringer Ingelheim International GmbH. (Germany)
- Bristol-Myers Squibb Company (U.S.)
- Bayer AG (Germany)
- Cipla Inc. (U.S.)
- Cadila Pharmaceuticals (India)
- Eli Lilly and Company (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- GlaxoSmithKline plc (U.K.)
- Johnson & Johnson Private Limited (U.S.)
- LEO Pharma A/S (Denmark)
- Lupin (India)
- Merck & Co., Inc. (U.S.)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Sumitomo Corporation (Japan)
- Teva Pharmaceutical Industries Ltd. (Israel)
Recent Developments in the Hepatitis C Market
Several recent developments have taken place in the field of Hepatitis C, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- On Oct 9, 2023, WHO Director-General Tedros Adhanom Ghebreyesus presented the Egyptian President Abdel Fattah El-Sisi with a certificate declaring that Egypt had been awarded gold tier status on the path to elimination of hepatitis C. Egypt, which had once had the highest prevalence of hepatitis C globally, is now the first country to have undergone validation for elimination by WHO and has achieved a milestone in disease elimination.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
13.6% |
|
Type |
|
|
Treatment |
|
|
Diagnosis |
|
|
Route of Administration |
|
|
End Users |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
