Age-Related Macular Degeneration (AMD) Disease - Anti VEGF Market Overview
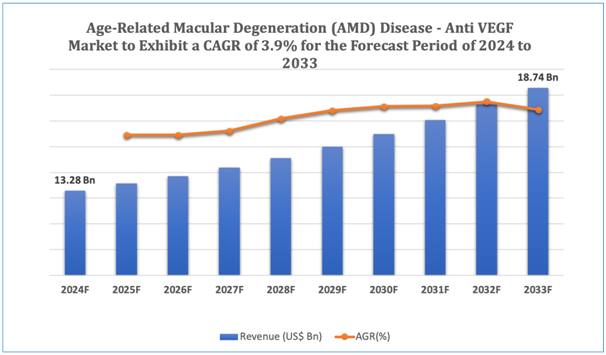
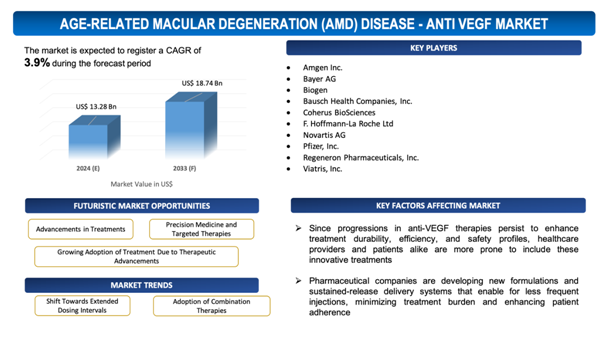
The global Age-Related Macular Degeneration (AMD) Disease - Anti VEGF market is estimated to be worth over USD 18.74Bn in 2033 and is expected to grow at CAGR of3.9% during the forecast period (2024-2033).
Age-Related Macular Degeneration (AMD) can be perceived asaadvanced eye illness that primarily affects older adults, leading to vision loss in the central part of the retina, known as the macula. Anti-VEGF (Vascular Endothelial Growth Factor) therapy has appeared as a foundation treatment for neovascular or "wet" AMD, the progressive form of the disease marked by aberrant blood vessel growth beneath the retina. Anti-VEGF drugs function by inhibiting the activity of VEGF, a protein that facilitates the growth of these unusual blood vessels, thusphasing down disease progression and conserving vision.
The global market for Anti-VEGF drugs in AMD has experiencedsubstantial growth, led by factors such as the growing prevalence of AMD, soaring awareness about the prominence of early diagnosis and treatment, and innovations in drug development and delivery technologies. Notable players in the pharmaceutical industry providenumerous Anti-VEGF drugs approved for the treatment of wet AMD, comprisingaflibercept, ranibizumab, and bevacizumab, which are administered via intravitreal injections. These drugs have illustratedefficiency in enhancing visual acuity and mitigating the risk of vision loss in patients with wet AMD, as it may lead to widespread adoption and incorporation into standard clinical practice. In addition, continuing research efforts emphasize on developing next-generation Anti-VEGF therapies with enhanceddurability, efficacy, and safety profiles, as well as exploring alternative treatment modalities such as gene therapy and continuous-release drug delivery systems. The global market for Anti-VEGF drugs in AMD is anticipated to continue soaring as the prevalence of AMD surges due to geriatric populations and lifestyle factors.
Along with that, breakthroughs in diagnostic techniques, such as optical coherence tomography (OCT) and genetic testing, are allowing earlier detection and personalized treatment approaches, further fueling market expansion. However, challenges such as the increased cost of treatment, the necessity for frequent injections, and the risk of complications associated with intravitreal injections remain substantial barriers to wider access and adoption of Anti-VEGF therapy for AMD. Nonetheless, continuous efforts to address these challenges and innovate in treatment alternatives hold commitment for enhancing outcomes and quality of life for individuals affected by AMD across the globe.
Figure 1. Age-Related Macular Degeneration (AMD) Disease - Anti VEGF: Market Size
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
AMD, a primary cause of vision loss among older adults, has spurred the demand for effective treatments to conserve vision and enhance patient outcomes. Anti-VEGF drugs have appeared as the standard of care for neovascular or "wet" AMD, limiting the unusual growth of blood vessels beneath the retina and hindering disease progression. Significant players in the pharmaceutical industry, comprising Novartis, Roche, and Regeneron Pharmaceuticals, dominate the market with approved Anti-VEGF drugs such as aflibercept (Eylea), ranibizumab (Lucentis), and bevacizumab (Avastin). These drugs are administered via intravitreal injections and have illustratedefficiency in enhancing visual acuity and mitigating the risk of vision loss in patients with wet AMD.
The current market panorama is marked by continuous research and development efforts focused at enhancing the durability, efficiency, and safety profiles of Anti-VEGF therapies. Major developments comprise the advent of longer-acting formulations and sustained-release drug delivery systems, providing the potential for extended treatment intervals and lessened treatment burden for patients. In addition to that, efforts to optimize treatment protocols and dosing regimens, such as treat-and-extend and personalized dosing approaches, focuson maximizing therapeutic benefits while reducing the need for frequent injections. Along with that, advancements in diagnostic techniques, such as optical coherence tomography (OCT) and genetic testing, allow earlier detection of AMD and personalized treatment strategies customized to individual patient characteristics.
Novel technologies, comprising gene therapy and gene editing approaches, hold hope for addressing the underlying mechanisms of AMD and offering long-term therapeutic benefits. In addition, the integration of artificial intelligence (AI) and machine learning algorithms into diagnostic and treatment algorithms improves precision medicine approaches and facilitates personalized care for patients with AMD. Overall, the global Anti-VEGF market for AMD stands ready for sustained growth and innovation, fuelled by advancements in drug development, delivery technologies, and personalized medicine approaches, at lengthenhancing outcomes for individuals affected by AMD globally.
Market Dynamics
Market Drivers
Increasing Incidence of Diseases &Surging Geriatric Population
AMD, a common cause of vision impairment and blindness among older adults, has witnessed a remarkablerise in prevalence attributing to factors such as geriatric populations and lifestyle variations. Since individuals age, they become more susceptible to developing AMD, specifically the neovascular or "wet" form, requiring effective treatment to avoid irreversible vision loss. In addition to that, the global geriatric population is expanding at a rapid pace, fueled by longer life expectancies and lessening birth rates, which may lead to a greater incidence of AMD and other age-associated eye diseases. This demographic trend creates a significant market opportunity for Anti-VEGF therapies, which have appeared as the standard of care for wet AMD. These therapies provide targeted inhibition of abnormal blood vessel growth beneath the retina, slowing disease progression and preserving vision. Since the prevalence of AMD continues to surge alongside the aging population, there is a growing demand for innovative treatments to address the soaring burden of AMD and improve patient outcomes. Pharmaceutical companies are actively investing in research and development efforts to improve existing Anti-VEGF therapies and develop next-generation treatments with enhanced efficacy and safety profiles. Along with that, advancements in diagnostic techniques and personalized medicine approaches enable earlier detection of AMD and tailored treatment strategies, further driving market growth. Overall, the increasing incidence of AMD and the growing geriatric population present compelling market opportunities for Anti-VEGF therapies, emphasizing the importance of continued innovation to meet the evolving needs of patients and healthcare systems across the world.
Market Restraints
With regard to numerous advantages of Age-Related Macular Degeneration (AMD) Disease - Anti VEGF, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- High Cost of Treatment: The increased cost associated with Anti-VEGF therapies for Age-Related Macular Degeneration (AMD) presents a substantial restraint on the global market.These drugs need frequent intravitreal injections, which are likely to resultin substantial treatment expenses over time.The financial burden of long-term therapy are likely to limit access to Anti-VEGF treatment for some patients, specifically those without adequate insurance coverage or financial resources.
- Treatment Burden and Compliance Challenges:The necessity for regular intravitreal injections poses as a notable treatment burden for patients with AMD receiving Anti-VEGF therapy.Patients are likely tosuffer from discomfort or anxiety associated with injections, that lead to poor treatment adherence and discontinuation.In addition, the requirement for frequent clinic visits for injections imposes logistical challenges for patients, particularly those residing in rural or underserved areas, hampering optimal treatment outcomes.
Market Opportunities
Growing Adoption of Treatment Due to Therapeutic Advancements
Sinceprogressions in anti-VEGF therapies persist to enhance treatment durability, efficiency, and safety profiles, healthcare providers and patients alike are more prone to include these innovative treatments. Novel formulations with extended dosing intervals and continuous-release drug delivery systems provide convenience and mitigate treatment burden for patients requiring regular injections. Along with that, improved understanding of AMD pathophysiology and personalized medicine approaches allow tailored treatment strategies, optimizing therapeutic outcomes. The soaring acceptance of Anti-VEGF therapies among ophthalmologists and retina specialists further triggers market growth, as they determine the benefits of these treatments in preserving vision and enhancing quality of life for patients with wet AMD. With continuous research and development efforts focused on further improvinganti-VEGF therapies, the market is poised for continued expansion as therapeutic advancements fuel increased adoption and acceptance of these innovative treatments in AMD management.
Market Trends
- Shift Towards Extended Dosing Intervals:
- A significant trend in the global anti-VEGF market for AMD is the move towards extended dosing intervals for treatment administration.
- Pharmaceutical companies are developing new formulations and sustained-release delivery systems that enable for less frequent injections, minimizing treatment burden and enhancing patient adherence.
- This trend focuseson enhancing convenience for patients and maximize treatment outcomes by ensuring consistent and effective inhibition of abnormal blood vessel growth beneath the retina.
- Adoption of Combination Therapies:
- Another appearing trend is the adoption of combination therapies in the management of AMD within the global anti-VEGF market.
- Healthcare providers are exploring the usage of anti-VEGF drugs in combination with other agents, such as steroids or angiogenesis inhibitors, to improve treatment efficacy and address diverse pathophysiological mechanisms.
- Combination therapies offer the potential for synergic effects, improved visual outcomes, and lessened treatment frequency, fuelling interest and research in this approach within the AMD treatment panorama.
Get more details on this report - Request Free Sample
Age-Related Macular Degeneration (AMD) Disease - Anti VEGF Market: Key Segments
By Product
- Eylea
- Lucentis
- Beovu
By Distribution Channel
- Hospitals
- Speciality Clinics
- Mail Order Pharmacies
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Age-Related Macular Degeneration (AMD) Disease - Anti VEGF Market: Regional Analysis
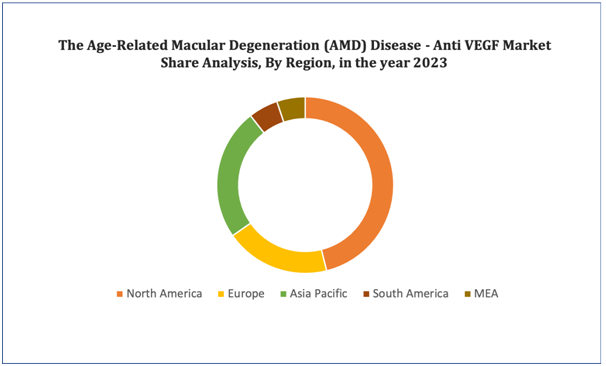
North America is the most significant global anti-VEGF therapeutics market shareholder. The growth of North America's anti-VEGF therapeutics market can be attributed to various factors, such as the high burden of disease and the increase in favorable government initiatives. Funding for research is high in the region. Moreover, increasing product launches and demand for medicines will positively impact the regional market's growth.
Figure 4. Age-Related Macular Degeneration (AMD) Disease - Anti VEGF Market: Distribution by Region
Get more details on this report - Request Free Sample
Leading Age-Related Macular Degeneration (AMD) Disease - Anti VEGF Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Age-Related Macular Degeneration (AMD) Disease - Anti VEGFmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Age-Related Macular Degeneration (AMD) Disease - Anti VEGFmarket is briefly discussed below.
The report includes the list of players operating in the global Age-Related Macular Degeneration (AMD) Disease - Anti VEGFmarket. Some of the key players include:
- Amgen Inc.
- Bayer AG
- Biogen
- Bausch Health Companies, Inc.
- Coherus BioSciences
- F. Hoffmann-La Roche Ltd
- Novartis AG
- Pfizer, Inc.
- Regeneron Pharmaceuticals, Inc.
- Viatris, Inc.
Recent Developments in the Age-Related Macular Degeneration (AMD) Disease - Anti VEGFMarket
Several recent developments have taken place in the field of Age-Related Macular Degeneration (AMD) Disease - Anti VEGF, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In January 202,Faricimab received its first approvals, in the USA, for use in the treatment of patients with neovascular (wet) age-related macular degeneration (nAMD) or diabetic macular edema (DME). Faricimab has also recently been approved in Japan, and is currently under regulatory review in the EU, for use in nAMD and DME. Phase III clinical development of Faricimab for use in the treatment of nAMD, DME, and macular edema due to retinal vein occlusion is continuing in multiple other countries worldwide.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
3.9% |
|
Product |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
