Adeno-Associated Virus (AAV) Vector-Based Gene Therapy Market Overview
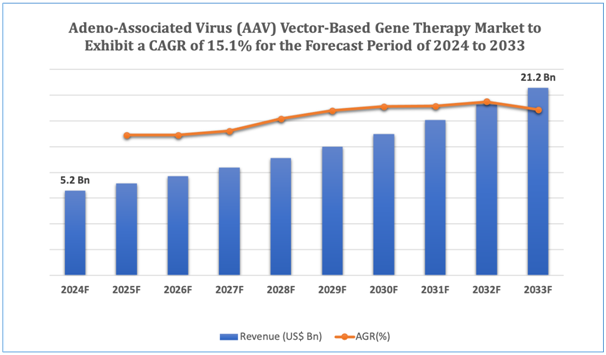
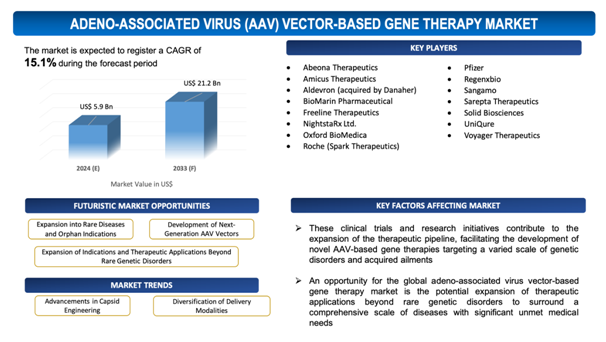
The global Adeno-Associated Virus (AAV) Vector-Based Gene Therapy market is estimated to be worth over USD 21.2Bn in 2033 and is expected to grow at CAGR of 15.1% during the forecast period (2024-2033).
Adeno-associated virus (AAV) vector-based gene therapy represents a favourable approach for treating a comprehensive range of genetic disorders and acquired diseases by delivering therapeutic genes into target cells. AAV vectors are acquired from non-pathogenic viruses and are specifically attractive for gene therapy owing to their low immunogenicity, capability to transduce both dividing and non-dividing cells, and long-term transgene expression. In AAV-based gene therapy, the therapeutic gene of interest is wrapped into the AAV vector, which is then delivered into the patient's cells, generally via intravenous infusion, intramuscular injection, or direct administration to target tissues. Once inside the cell, the AAV vector delivers the therapeutic gene, enabling it to be expressed and potentially correct the underlying genetic defect or offer a therapeutic benefit.
The global market for AAV vector-based gene therapy has experiencedstrong growth in the past few years, fueled by several key factors. One significant factor is the growing prevalence of genetic disorders and chronic diseases for which traditional treatments are inadequate or non-existent. AAV-based gene therapy provides the potential to tackle the primary cause of these conditions by providing long-lasting or permanent expression of therapeutic genes. In addition to that, advancements in biotechnology and gene delivery techniques have promoted the development of safer and more efficient AAV vectors, improving their therapeutic potential and diversifying their applicability across a thorough range of diseases.
Furthermore, the soaring investment and interest from pharmaceutical firms, biotechnology companies, and research institutions have propelled the development and commercialization of AAV-based gene therapies. This has paved its way to a pipeline of promising candidates in different stages of clinical development targeting a diverse range of diseases, comprising inherited genetic disorders, neurological diseases, and variouskinds of cancer. With regulatory approvals for AAV-based gene therapies increasing and ongoing efforts to optimize manufacturing processes and mitigate production costs, the global market for AAV vector-based gene therapy is poised for continued expansion. As more therapies enter the market and illustrate clinical efficiency, AAV-based gene therapy holds immense promise for revolutionizing the treatment panorama for several genetic and acquired diseases, offering commitment to patients and healthcare providers across the world.
Figure 1. Adeno-Associated Virus (AAV) Vector-Based Gene Therapy: Market Size
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global adeno-associated virus (AAV) vector-based gene therapy market is experiencingstrong growth and evolution, fueled by substantial market insights and innovations in technology. Key insights reveal a growing demand for advanced treatments for genetic disorders and chronic diseases, accelerating the development and adoption of AAV-based gene therapies. Significant developments comprise the expansion of the therapeutic pipeline, with an emphasis on addressing unmet medical necessitates across a range of diseases, comprising neurodegenerative diseases, rare genetic disorders, and variouskinds of cancer. In addition to that, advancements in AAV vector engineering and manufacturing technologies have improved the efficiency, safety, and scalability of gene therapy platforms, supporting broader clinical application and commercialization. Novel technologies such as capsid engineering, tissue-specific targeting, and gene editing techniques are driving innovation in AAV-based gene therapy, allowing precise and effective delivery of therapeutic genes to target tissues and cells. With growing regulatory approvals and investment from pharmaceutical firms and biotechnology firms, the current market landscape for AAV vector-based gene therapy is marked by soaring momentum and optimism, providingrevolutionary treatment options for patients across the world.
Market Dynamics
Market Drivers
Increasing Number of Clinical Trials and Research Activities in the Gene Therapy Space
These clinical trials and research initiatives contribute to the expansion of the therapeutic pipeline, facilitating the development of novel AAV-based gene therapies targeting a variedscale of genetic disorders and acquired ailments. As more gene therapy candidates progress through clinical testing and illustrate efficacy and safety profiles, investor confidence in the potential of AAV-based gene therapy increases, resultingin greater investment and commercialization efforts.
In addition to that, clinical trials offer valuable insights into the mechanisms of action, dosing regimens, and patient responses to AAV vector-based gene therapies, supporting optimization of treatment strategies and regulatory approval processes. The increasing body of clinical evidence facilitating the effectiveness of AAV-based gene therapies propels market expansion by inculcatingconviction among healthcare providers, patients, and regulatory authorities, fueling adoption and market penetration. Overall, the growing number of clinical trials and research activities in the gene therapy space propels innovation and fuels the growth of the global AAV vector-based gene therapy market, providing transformative treatment alternatives for patients with genetic disorders and chronic ailments.
Market Restraints
With regard to numerous advantages of Adeno-Associated Virus (AAV) Vector-Based Gene Therapy, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- Immune Response and Immunogenicity: One market restraint for the global AAV vector-based gene therapy market is the potential for immune responses and immunogenicity against the AAV vector or the therapeutic transgene. Pre-existing immunity to AAV vectors or immune responses triggered by vector administration can restrict the efficiency and durability of gene therapy treatments, especially upon repeat dosing. Addressing immune-related challenges remains a considerable hurdle in optimizing the safety and long-term efficacy of AAV-based gene therapies.
- Limited Vector Capacity and Payload Constraints: Another market restraint is the limited cargo capacity of AAV vectors and payload constraints related to packaging therapeutic genes. AAV vectors have a restricted carrying capacity for genetic material, confining the size of transgenes that can be delivered. This constraint poses challenges for the treatment of disorders requiring the delivery of large or multiple therapeutic genes. In addition to that, optimizing vector design and payload delivery strategies to accommodate larger genes or multiple gene combinations without compromising vector stability or transduction efficiency remains a technical barrier in AAV-based gene therapy development.
Market Opportunities
Expansion of Indications and Therapeutic Applications Beyond Rare Genetic Disorders
An opportunity for the global adeno-associated virus vector-based gene therapy market is the potential expansion of therapeutic applications beyond rare genetic disorders to surround a comprehensivescale of diseases with significant unmet medical needs. AAV vectors provide a versatile and effective platform for delivering therapeutic genes to target tissues, making them promising candidates for addressing complicated and multifactorial diseases such as cardiovascular diseases, neurodegenerative disorders, and various kinds of cancer. With innovations in vector design, manufacturing processes, and delivery techniques, AAV-based gene therapies are becoming increasingly capable of addressing the underlying pathophysiology of these diseases and offering long-lasting therapeutic benefits. In addition to that, the advent of gene editing technologies, coupled with AAV vectors, offers hope for developing personalized therapies tailored to individual patient profiles and disease characteristics. By diversifying the therapeutic panorama to comprise a broader spectrum of diseases, the global AAV vector-based gene therapy market stands to capitalize on new opportunities for innovation, investment, and market growth, ultimately transforming the treatment paradigm for a thorough range of debilitating conditions and enhancing patient outcomes on a worldwide scale.
Market Trends
- Advancements in Capsid Engineering: A significant trend in the global AAV vector-based gene therapy market is the continuing advancements in capsid engineering techniques. Capsids are the outer protein shells of AAV vectors responsible for cellular entry and transgene delivery. By engineering capsids for improved tissue tropism, improved transduction efficiency, and reduced immunogenicity, researchers focuson optimizing vector performance and widen the therapeutic applicability of AAV-based gene therapies.
- Diversification of Delivery Modalities: Another substantial trend is the expansion of delivery modalities for AAV vector-based gene therapies. While conventional routes such as intravenous infusion or intramuscular injection remain common, there is soaring interest in exploring alternative delivery approaches, including intrathecal administration for targeting the central nervous system, direct injection into specific tissues or organs, and ex vivo gene therapy methods. This trend indicates efforts to optimize delivery strategies to maximize therapeutic efficacy, minimize off-target effects, and widen the scope of treatable diseases with AAV-based gene therapies.
Get more details on this report - Request Free Sample
Adeno-Associated Virus (AAV) Vector-Based Gene Therapy Market: Key Segments
By Gene Therapy Application
- Neurological Disorders
- Ophthalmic Diseases
- Muscular Disorders
- Hematological Disorders
By Adeno-Associated Virus Serotype
- AAV1
- AAV2
- AAV5
- AAV9
By End-User
- Hospitals
- Research Institutes
- Biopharmaceutical Companies
By Therapeutic Area
- Rare Diseases
- Oncology
- Cardiovascular Diseases
- Genetic Disorders
By Distribution Channel
- Direct Tender
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Adeno-Associated Virus (AAV) Vector-Based Gene Therapy Market: Regional Analysis
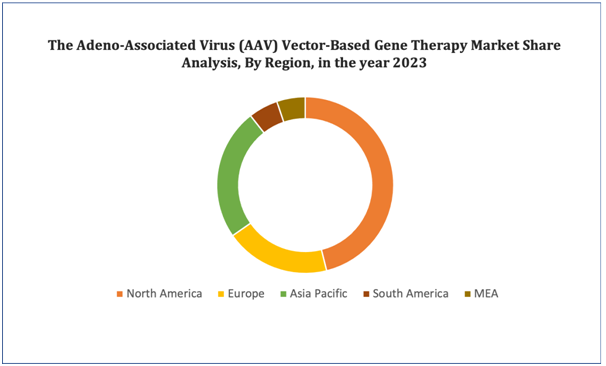
North America market growth is driven majorly by revenue contribution from the United States, which has been a prominent player in the gene therapy sector. Factors such as robust research and development activities, supportive regulatory environment, and substantial investments contribute to the North America leading among the other regional markets. Europe market revenue growth is supported by substantial contribution from countries such as the United Kingdom and Germany, which also have been demonstrated significant growth in gene therapy research and development. Asia Pacific market growth is driven by rising investments in biotechnology and healthcare infrastructure in China and Japan.
Figure 4. Adeno-Associated Virus (AAV) Vector-Based Gene Therapy Market: Distribution by Region
Get more details on this report - Request Free Sample
Leading Adeno-Associated Virus (AAV) Vector-Based Gene Therapy Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Adeno-Associated Virus (AAV) Vector-Based Gene Therapy (ARDS) market, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Adeno-Associated Virus (AAV) Vector-Based Gene Therapymarket are briefly discussed below.
The report includes the list of players operating in the global Adeno-Associated Virus (AAV) Vector-Based Gene Therapymarket. Some of the key players include:
- Abeona Therapeutics
- Amicus Therapeutics
- Aldevron (acquired by Danaher)
- BioMarin Pharmaceutical
- Freeline Therapeutics
- NightstaRx Ltd.
- Oxford BioMedica
- Roche (Spark Therapeutics)
- Pfizer
- Regenxbio
- Sangamo
- Sarepta Therapeutics
- Solid Biosciences
- UniQure
- Voyager Therapeutics
Recent Developments in the Adeno-Associated Virus (AAV) Vector-Based Gene Therapy Market
Several recent developments have taken place in the field of Adeno-Associated Virus (AAV) Vector-Based Gene Therapy, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In March 2023, Charles River Laboratories International, Inc. announced the launch of its off-the-shelf pHelper offering, which is designed to secure supply and streamline adeno-associated virus (AAV)-based gene therapy programs from early discovery through commercial manufacturing. It is available immediately in Research Grade (RG), High Quality (HQ), and Good Manufacturing Practice (GMP)-grade.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
15.1% |
|
Gene Therapy Application |
|
|
Adeno-Associated Virus Serotype |
|
|
End User |
|
|
Therapeutic Area |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
